- Archeologists can determine the age of organic matter by measuring the proportion of carbon-14 present in a sample. Assuming that carbon-14 has a half- life...(Solved)
Archeologists can determine the age of organic matter by measuring the proportion of carbon-14 present in a sample. Assuming that carbon-14 has a half- life of 5600 years, calculate the age of a piece of wood found to contain 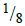 as much carbon-14 as in a living material.
as much carbon-14 as in a living material.
Date posted: August 14, 2019. Answers (1)
- Explain the observation made in the experiment whose set up is shown below.(Solved)
Explain the observation made in the experiment whose set up is shown below.
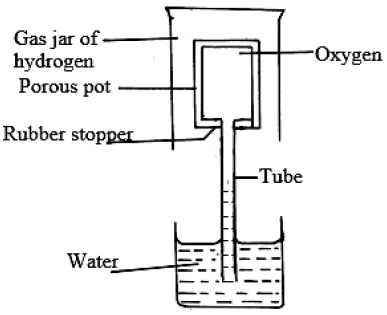
Date posted: August 14, 2019. Answers (1)
- Element G and T have atomic number 20 and 9, respectively.(Solved)
Element G and T have atomic number 20 and 9, respectively.
(a) Write down the electron arrangement of the ion of G.
(b) Which element has the same electron arrangement as the ion of T?
(c) Write the formula of compound formed by reaction of G and T.
Date posted: August 14, 2019. Answers (1)
- An Element X has first, second and third ionization energies. State and explain how their trends compare.(Solved)
An Element X has first, second and third ionization energies. State and explain how their trends compare.
Date posted: August 5, 2019. Answers (1)
- Why is an atom said to be electrically neutral?(Solved)
Why is an atom said to be electrically neutral?
Date posted: August 5, 2019. Answers (1)
- Name one ore of Zinc metal(Solved)
Name one ore of Zinc metal
Date posted: August 5, 2019. Answers (1)
- A sample of a colorless solution is suspected to be Zinc (II) sulphate. Describe some tests that can be carried to prove this.(Solved)
A sample of a colorless solution is suspected to be Zinc (II) sulphate. Describe some tests that can be carried to prove this.
Date posted: August 5, 2019. Answers (1)
- Calculate the percentage of nitrogen in calcium nitrate(Solved)
Calculate the percentage of nitrogen in calcium nitrate
Date posted: August 5, 2019. Answers (1)
- Dry carbon (II) oxide gas reacts with hot lead (II) oxide as shown in the equation below.(Solved)
Dry carbon (II) oxide gas reacts with hot lead (II) oxide as shown in the equation below.
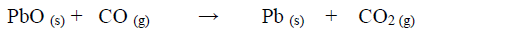
a) Name another gas that can be used to function as carbon (II) oxide in this experiment.
b) With an appropriate reason, identify the oxidizing agent in the equation above.
Date posted: August 5, 2019. Answers (1)
- Highlight one precaution observed in each of the following cases:
i. When evaporating Ethanol.
ii. When heating to dryness a hydrated salts.(Solved)
Highlight one precaution observed in each of the following cases:
i. When evaporating Ethanol.
ii. When heating to dryness a hydrated salts.
Date posted: August 5, 2019. Answers (1)
- A metal Y with atomic number 11 burns in chlorine to produce a white solid X.Describe the following properties of X in terms of
i)...(Solved)
A metal Y with atomic number 11 burns in chlorine to produce a white solid X.Describe the following properties of X in terms of
i) Solubility.
ii).Electrical conductivity
Date posted: August 5, 2019. Answers (1)
- Excess chlorine was bubbled through a solution of potassium bromide. State and explain the observation made.(Solved)
Excess chlorine was bubbled through a solution of potassium bromide. State and explain the observation made.
Date posted: August 5, 2019. Answers (1)
- Why is cryolite added to the pure Aluminium oxide in the process of extracting the metal?.(Solved)
Why is cryolite added to the pure Aluminium oxide in the process of extracting the metal?.
Date posted: August 5, 2019. Answers (1)
- The table below gives some information about four elements. The letters are not their actual symbols.(Solved)
The table below gives some information about four elements. The letters are not their actual symbols.

i. Write the electron arrangement of any element in same chemical family as element L.
ii. Compare the reactivity of elements K and N.
iii. Account for the difference in ionic and atomic radii of element M.
Date posted: August 5, 2019. Answers (1)
- Give two reasons why spoons are electroplated.(Solved)
Give two reasons why spoons are electroplated.
Date posted: August 5, 2019. Answers (1)
- An indicator established the following equilibrium when dissolved in water.(Solved)
An indicator established the following equilibrium when dissolved in water.

State and explain the observation made when Lime water is added?
Date posted: August 5, 2019. Answers (1)
- Solutions may be classified as strong basic, weakly acidic, strong acidic. The information below gives solutions and their PH values. Study it and answer the...(Solved)
Solutions may be classified as strong basic, weakly acidic, strong acidic. The information below gives solutions and their PH values. Study it and answer the questions that follow.

i).Classify the solutions in the table above using terms above
ii).Which ions are pre-dominantly in solution C?
Date posted: August 5, 2019. Answers (1)
- Explain why there is effervescence when lemon juice is added to sodium hydrogen carbonate. ...(Solved)
Explain why there is effervescence when lemon juice is added to sodium hydrogen carbonate.
Date posted: August 5, 2019. Answers (1)
- The diagram below represents the Haber process for the manufacture of ammonia. Study it and answer the questions that follow.(Solved)
The diagram below represents the Haber process for the manufacture of ammonia. Study it and answer the questions that follow.

a) Name any two impurities removed by the purifier.
b) The catalyst used in the process is finely divided iron. Why iron is finely divided?
c) In the Haber process the conversion of nitrogen and hydrogen into ammonia is only 10%.
The remaining unreacted gases are recycled. What is the advantage of this.
d) A part from iron catalyst and pressure of 500 atmospheres, name any other condition required for this process.
Date posted: August 5, 2019. Answers (1)
- The table below gives some elements of the periodic table and their atomic masses, atomic numbers and melting points. The letters are not the actual...(Solved)
The table below gives some elements of the periodic table and their atomic masses, atomic numbers and melting points. The letters are not the actual symbols of the elements.

(a) Select two elements with oxidation states of -3.
(b) Which elements represent the most powerful reducing agent? Explain.
(c) Which element has the highest ionization energy?
(d) Select two elements which when reacted form a compound that conducts electricity both in molten and aqueous state.
(e) Select any two elements which when reacted form a compound that dissolves in water to form an acidic solution.
(f) Using dots (●) and crosses (X) to represent electrons; draw diagrams to show bonding between B and J.
(g) Explain why for some elements the atomic mass is not twice the atomic number.
(h) Explain why the melting point of element K is higher than that of element D.
(i) Describe how a solid mixture of the Sulphate of element K and lead (II) Sulphate can be separated.
Date posted: August 5, 2019. Answers (1)