- Form one students wanted to prepare hydrogen gas in the laboratory. They reacted Zinc granules and dilute nitric (V) acid.(Solved)
Form one students wanted to prepare hydrogen gas in the laboratory. They reacted Zinc granules and dilute nitric (V) acid.
Explain the observation made
Date posted: August 16, 2019. Answers (1)
- If a factory produces 1000 kg of sodium hydroxide in every 24 hours, calculate the required current per day. (H= 1.0, Na = 23.0, O...(Solved)
If a factory produces 1000 kg of sodium hydroxide in every 24 hours, calculate the required current per day. (H= 1.0, Na = 23.0, O = 16.0)
Date posted: August 16, 2019. Answers (1)
- The diagram below represents a mercury cell chlor-alkali process that can be used in the industrial manufacture of sodium hydroxide. Study it and answer the...(Solved)
The diagram below represents a mercury cell chlor-alkali process that can be used in the industrial manufacture of sodium hydroxide. Study it and answer the questions that follow.
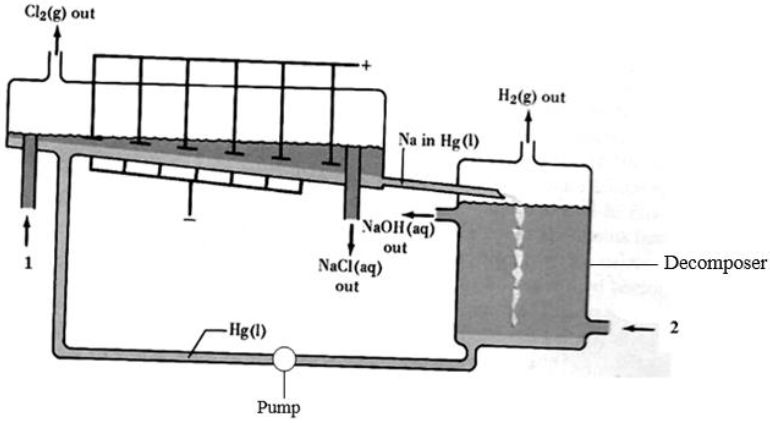
(a) (i) Name the raw materials introduced at 1 and 2.
(ii) Identify a substance that can be used as anode.
(iii) Write equations for the reactions taking place at:
Cathode
Decomposer
(iv) How is the aqueous sodium hydroxide purified?
Date posted: August 16, 2019. Answers (1)
- The structure below is a polyamide formed from two types of monomers.(Solved)
The structure below is a polyamide formed from two types of monomers.
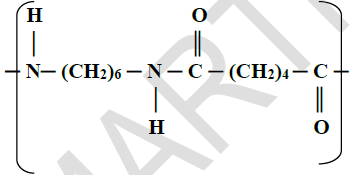
(i) Give the structural formula of the two monomers forming the polyamide above.
(ii) Name the type of polymerisation.
(iii) Give one use of the polyamide.
Date posted: August 16, 2019. Answers (1)
- The table below shows some properties of the organic compounds U, V and W. Use the information to answer questions that follow.(Solved)
The table below shows some properties of the organic compounds U, V and W. Use the information to answer questions that follow.

To which homologous series does each compound belong?
U
V
W
Date posted: August 16, 2019. Answers (1)
- The scheme below shows several reactions starting with propanol. Study the scheme and answer the questions which follow.(Solved)
The scheme below shows several reactions starting with propanol. Study the scheme and answer the questions which follow.

(a) (i) Name gas L.
(ii) Name and draw the structural formula of compound K.
(iii) What conditions and reagents are necessary to convert M and N?
Reagents
Conditions
(iv) Write chemical equation for the conversion of K to J.
Date posted: August 16, 2019. Answers (1)
- Given that:
Enthalpy of combustion of Carbon (graphite) = -393kJmol-1
Enthalpy of combustion of carbon (II) oxide = -283 kJmol-1(Solved)
Given that:
Enthalpy of combustion of Carbon (graphite) = -393kJmol-1
Enthalpy of combustion of carbon (II) oxide = -283 kJmol-1
(a) Write down the thermo-chemical equations for the combustion of:
(i) Carbon (graphite)
(ii) Carbon (II) oxide
(b) Determine the enthalpy of formation of carbon (II) oxide.
(c) Draw an energy level diagram for the processes in (a) and (b) above.
(d) State two properties of Carbon (IV) Oxide that make it suitable for use in fire extinguishers.
(e) When 8.0 g of sulphur is completely burned in oxygen in a calorimeter, the heat evolved raises the temperature of 500 cm3 of water by 35 0C. Calculate the heat of combustion of sulphur.
(The specific heat capacity of water = 
Date posted: August 16, 2019. Answers (1)
- Below is radioactive decay series starting from
 and ending at
and ending at  (Solved)
(Solved)
Below is radioactive decay series starting from  and ending at
and ending at 

(i) Identify the radiations emitted at step I and step II.
(ii) Write nuclear equation for reaction which takes place at step III.
(iii) State one way in which radioisotopes poses danger to the environment.
Date posted: August 16, 2019. Answers (1)
- The diagram below shows some properties of nuclear radiations.(Solved)
The diagram below shows some properties of nuclear radiations.

Identify the radiation represented by letter Q. Give reason.
Date posted: August 16, 2019. Answers (1)
- The table below shows the radioactive decay for a sample of iodine -131.Study it and answer the questions that follow.(Solved)
The table below shows the radioactive decay for a sample of iodine -131.Study it and answer the questions that follow.

(i) Plot the decay curve of iodine -131 using the data given in the table above.
(ii) Use the graph to determine the half-life of the sample.
(iii) What fraction of the original sample remains after 18 days?
Date posted: August 16, 2019. Answers (1)
- Study the diagram below and answer the questions that follow.(Solved)
Study the diagram below and answer the questions that follow.

(i) State the observation made in the lime water. Explain.
(ii) How can the identity of substance K be demonstrated?
Date posted: August 16, 2019. Answers (1)
- In an experiment to determine the proportion of oxygen in air, Copper turning were packed in excess in a combustion tube connected to two syringes...(Solved)
In an experiment to determine the proportion of oxygen in air, Copper turning were packed in excess in a combustion tube connected to two syringes of 80 cm3 each in a volume . Syringe R contained 80 cm3 of air while syringe S was closed and empty as shown.

Air was passed over heated turnings slowly and repeatedly until there was no further change in volume. After cooling, 63.2 cm3 of air remained in syringe R.
(i) Why was copper packed in excess?
(ii) State one observation made in the combustion tube during experiment.
(iii) Give an equation for the reaction that took place in combustion tube.
(iv) Determine the percentage of oxygen used up during the experiment.
Date posted: August 16, 2019. Answers (1)
- The flow chart below illustrates two industrial processes. Study it and answer the questions that follow.(Solved)
The flow chart below illustrates two industrial processes. Study it and answer the questions that follow.

(a) Name of the process by which air is separated into oxygen and nitrogen.
(b) Identify the substances which are represented by the letters.
A
B
C
D
E
F
(c) (i) Name the catalyst involved in the formation of A and E.
(ii) Explain the role of the catalysts named in (c) above.
(d) (i) Write the chemical equation for the reaction taking place in the absorption tower.
(ii) Why is it not advisable to use hard water to produce sulphuric acid?
(e) (i) What property of concentrated sulphuric acid makes it suitable for the preparation of nitric (v) acid and hydrogen chloride?
(ii) Write chemical equations for the reaction of zinc metal with dilute sulphuric acid and concentrated sulphuric acid.
Dilute sulphuric acid
Concentrated sulphuric acid
Date posted: August 16, 2019. Answers (1)
- In an experiment to separate a mixture of water (100oC) and ethanol (78.4oC), a student set up the apparatus shown below.(Solved)
In an experiment to separate a mixture of water (100oC) and ethanol (78.4oC), a student set up the apparatus shown below.

(a) Identify the mistakes in the set up.
(b) What method would the student use to test the purity of ethanol obtained?
Date posted: August 16, 2019. Answers (1)
- Ammonia nitrate is one of the nitrogenous fertilizers manufactured from nitrogen compounds.(Solved)
Ammonia nitrate is one of the nitrogenous fertilizers manufactured from nitrogen compounds.
(a) Identify the reagents used to manufacture ammonium nitrate.
(b) Calculate the percentage of nitrogen in ammonium nitrate fertilizer.(N = 14, H = 1, O =16)
Date posted: August 14, 2019. Answers (1)
- Nitrogen may be isolated from air or from the reaction between ammonium chloride and sodium nitrite.(Solved)
Nitrogen may be isolated from air or from the reaction between ammonium chloride and sodium nitrite.
(a) (i) State the condition necessary for the production of nitrogen from ammonium chloride and sodium nitrite.
(ii) Write an equation for the reaction above.
(b) Name a major impurity contained in nitrogen isolated from air.
Date posted: August 14, 2019. Answers (1)
- Calculate the number of aluminum ions in 250 cm3 of 0.1 M aluminium sulphate (Avogadro’s Constant = 6.0 × 1023)(Solved)
Calculate the number of aluminum ions in 250 cm3 of 0.1 M aluminium sulphate (Avogadro’s Constant = 6.0 × 1023)
Date posted: August 14, 2019. Answers (1)
- Study the information given below for magnesium and calcium.(Solved)
Study the information given below for magnesium and calcium.

(a) Explain the trend in ionisation energies between magnesium and calcium.
(b) Explain why the two elements have higher 2nd ionisation energies than the 1st one.
Date posted: August 14, 2019. Answers (1)
- Use the bond energy values given below to determine the enthalpy change for the conversion of propene to propane by hydrogenation.(Solved)
Use the bond energy values given below to determine the enthalpy change for the conversion of propene to propane by hydrogenation.

Date posted: August 14, 2019. Answers (1)
- Using reagents provided, explain how you could prepare dry zinc carbonate solid.
Zinc powder, dilute nitric (V) acid, Water and Solid sodium carbonate(Solved)
Using reagents provided, explain how you could prepare dry zinc carbonate solid.
Zinc powder, dilute nitric (V) acid, Water and Solid sodium carbonate
Date posted: August 14, 2019. Answers (1)