Chlorine 35
Chlorine 37
maurice.mutuku answered the question on August 16, 2019 at 14:32
- Study the set up below and answer the questions that follow.(Solved)
Study the set up below and answer the questions that follow.
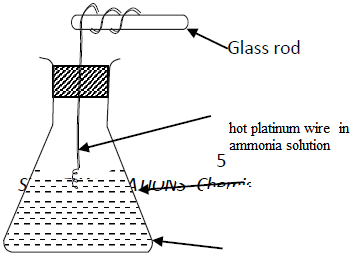
(a) State and explain the observations made in the conical.
(b) Write an equation for the reaction that takes place in the conical flask.
Date posted: August 16, 2019. Answers (1)
- Using an energy cycle diagram, determine the enthalpy formation of ethanol, given that:(Solved)
Using an energy cycle diagram, determine the enthalpy formation of ethanol, given that:
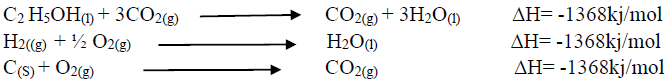
Date posted: August 16, 2019. Answers (1)
- During the manufacture of sodium carbonate by solvay process,there is a by-product produced which is not recycled.(Solved)
During the manufacture of sodium carbonate by solvay process,there is a by-product produced which is not recycled.
(a) Write the equation for the formation of this by-product.
(b) State one of the uses of the product (a) above.
(c) State one use of the sodium carbonate formed.
Date posted: August 16, 2019. Answers (1)
- With explanation, state whether the following equation has undergone an oxidation or reduction process.(Solved)
With explanation, state whether the following equation has undergone an oxidation or reduction process.

Date posted: August 16, 2019. Answers (1)
- A piece of cheese contains proteins which are made up of amino acid as shown.(Solved)
A piece of cheese contains proteins which are made up of amino acid as shown.

(a) Name the type of polymerization involved in protein formation.
(b) Show the structural formula for the polymer formed when the amino acids polymerise.
Date posted: August 16, 2019. Answers (1)
- Using dots and crosses, show the type of bonding in Calcium Chloride(Solved)
Using dots and crosses, show the type of bonding in Calcium Chloride
Date posted: August 16, 2019. Answers (1)
- A student accidentally mixed three solids: lead (II) sulphate, copper (II) oxide and Iron (III) chloride. Describe how you would obtain a solid lead (II)...(Solved)
A student accidentally mixed three solids: lead (II) sulphate, copper (II) oxide and Iron (III) chloride. Describe how you would obtain a solid lead (II) sulphate from the mixture.
Date posted: August 16, 2019. Answers (1)
- When a few drops of sodium hydroxide solution were added to a colorless test solution, a white precipitate was formed. When excess sodium hydroxide solution...(Solved)
When a few drops of sodium hydroxide solution were added to a colorless test solution, a white precipitate was formed. When excess sodium hydroxide solution was added, the precipitate dissolved. Similar results were obtained with the test solution when aqueous ammonia was used.
(a) Identify the cation present in the test solution.
(b) Identify the precipitate formed with the few drops of sodium hydroxide solution were added
(c) Write a balanced ionic equation for the reaction between the precipitate and excess ammonia solution.
Date posted: August 16, 2019. Answers (1)
- Explain the effect of hydrogen chloride on blue and red litmus papers when it is dissolved in water and in methylbenzene.(Solved)
Explain the effect of hydrogen chloride on blue and red litmus papers when it is dissolved in water and in methylbenzene.
Date posted: August 16, 2019. Answers (1)
- Consider the following equilibrium reaction.(Solved)
Consider the following equilibrium reaction.

Identify the acid and base in the forward reaction. Explain your answer
Date posted: August 16, 2019. Answers (1)
- Form one students wanted to prepare hydrogen gas in the laboratory. They reacted Zinc granules and dilute nitric (V) acid.(Solved)
Form one students wanted to prepare hydrogen gas in the laboratory. They reacted Zinc granules and dilute nitric (V) acid.
Explain the observation made
Date posted: August 16, 2019. Answers (1)
- If a factory produces 1000 kg of sodium hydroxide in every 24 hours, calculate the required current per day. (H= 1.0, Na = 23.0, O...(Solved)
If a factory produces 1000 kg of sodium hydroxide in every 24 hours, calculate the required current per day. (H= 1.0, Na = 23.0, O = 16.0)
Date posted: August 16, 2019. Answers (1)
- The diagram below represents a mercury cell chlor-alkali process that can be used in the industrial manufacture of sodium hydroxide. Study it and answer the...(Solved)
The diagram below represents a mercury cell chlor-alkali process that can be used in the industrial manufacture of sodium hydroxide. Study it and answer the questions that follow.
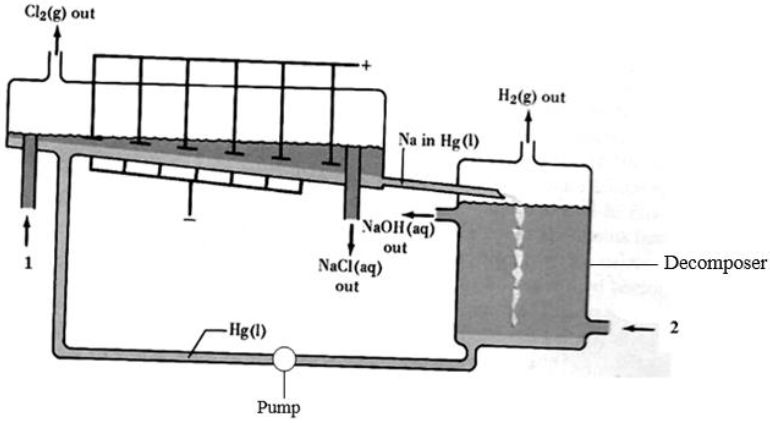
(a) (i) Name the raw materials introduced at 1 and 2.
(ii) Identify a substance that can be used as anode.
(iii) Write equations for the reactions taking place at:
Cathode
Decomposer
(iv) How is the aqueous sodium hydroxide purified?
Date posted: August 16, 2019. Answers (1)
- The structure below is a polyamide formed from two types of monomers.(Solved)
The structure below is a polyamide formed from two types of monomers.
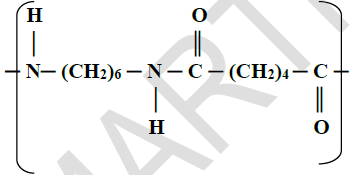
(i) Give the structural formula of the two monomers forming the polyamide above.
(ii) Name the type of polymerisation.
(iii) Give one use of the polyamide.
Date posted: August 16, 2019. Answers (1)
- The table below shows some properties of the organic compounds U, V and W. Use the information to answer questions that follow.(Solved)
The table below shows some properties of the organic compounds U, V and W. Use the information to answer questions that follow.

To which homologous series does each compound belong?
U
V
W
Date posted: August 16, 2019. Answers (1)
- The scheme below shows several reactions starting with propanol. Study the scheme and answer the questions which follow.(Solved)
The scheme below shows several reactions starting with propanol. Study the scheme and answer the questions which follow.

(a) (i) Name gas L.
(ii) Name and draw the structural formula of compound K.
(iii) What conditions and reagents are necessary to convert M and N?
Reagents
Conditions
(iv) Write chemical equation for the conversion of K to J.
Date posted: August 16, 2019. Answers (1)
- Given that:
Enthalpy of combustion of Carbon (graphite) = -393kJmol-1
Enthalpy of combustion of carbon (II) oxide = -283 kJmol-1(Solved)
Given that:
Enthalpy of combustion of Carbon (graphite) = -393kJmol-1
Enthalpy of combustion of carbon (II) oxide = -283 kJmol-1
(a) Write down the thermo-chemical equations for the combustion of:
(i) Carbon (graphite)
(ii) Carbon (II) oxide
(b) Determine the enthalpy of formation of carbon (II) oxide.
(c) Draw an energy level diagram for the processes in (a) and (b) above.
(d) State two properties of Carbon (IV) Oxide that make it suitable for use in fire extinguishers.
(e) When 8.0 g of sulphur is completely burned in oxygen in a calorimeter, the heat evolved raises the temperature of 500 cm3 of water by 35 0C. Calculate the heat of combustion of sulphur.
(The specific heat capacity of water = 
Date posted: August 16, 2019. Answers (1)
- Below is radioactive decay series starting from
 and ending at
and ending at  (Solved)
(Solved)
Below is radioactive decay series starting from  and ending at
and ending at 

(i) Identify the radiations emitted at step I and step II.
(ii) Write nuclear equation for reaction which takes place at step III.
(iii) State one way in which radioisotopes poses danger to the environment.
Date posted: August 16, 2019. Answers (1)
- The diagram below shows some properties of nuclear radiations.(Solved)
The diagram below shows some properties of nuclear radiations.

Identify the radiation represented by letter Q. Give reason.
Date posted: August 16, 2019. Answers (1)
- The table below shows the radioactive decay for a sample of iodine -131.Study it and answer the questions that follow.(Solved)
The table below shows the radioactive decay for a sample of iodine -131.Study it and answer the questions that follow.

(i) Plot the decay curve of iodine -131 using the data given in the table above.
(ii) Use the graph to determine the half-life of the sample.
(iii) What fraction of the original sample remains after 18 days?
Date posted: August 16, 2019. Answers (1)