- Draw a well labelled diagram to show the penetrating power of the three types of nuclear radiations.(Solved)
(a) Draw a well labelled diagram to show the penetrating power of the three types of nuclear radiations.
(b) . Which radiation causes more harm to human cells? Explain.
Date posted: August 19, 2019. Answers (1)
- Identify the following substances.(Solved)
Identify the following substances.
(a) (i) 
(ii) 
(b)Which of the above substances is suitable for use with hard water? Explain.
Date posted: August 19, 2019. Answers (1)
- Alkanoic acids have a higher boiling point than the corresponding alkanols.(Solved)
Alkanoic acids have a higher boiling point than the corresponding alkanols. Explain this
Date posted: August 19, 2019. Answers (1)
- Write an equation for the reaction when copper pyrite is roasted in air.(Solved)
Write an equation for the reaction when copper pyrite is roasted in air.
Date posted: August 19, 2019. Answers (1)
- Use the cell representation below to answer the question that follows.(Solved)
Use the cell representation below to answer the question that follows.

Date posted: August 16, 2019. Answers (1)
- Consider the following equilibrium reaction.(Solved)
Consider the following equilibrium reaction.
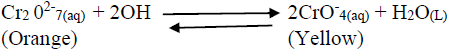
State and explain what would be observed when dilute hydrochloric acid is added into the equilibrium mixture.
Date posted: August 16, 2019. Answers (1)
- 28g of a saturated solution of a salt at 250 C yielded 10g of solid when evaporated to dryness. Calculate the solubility of the salt...(Solved)
28g of a saturated solution of a salt at 250 C yielded 10g of solid when evaporated to dryness. Calculate the solubility of the salt at 250 C
Date posted: August 16, 2019. Answers (1)
- Study the flow diagram below answer the questions that follow.(Solved)
Study the flow diagram below answer the questions that follow.

(a) Name liquid L
(b) Write an ionic equation for the formation of Solid X
Date posted: August 16, 2019. Answers (1)
- Using equations, distinguish between the bleaching action of chlorine and Sulphur (IV) oxide.(Solved)
Using equations, distinguish between the bleaching action of chlorine and Sulphur (IV) oxide.
Date posted: August 16, 2019. Answers (1)
- Identify the two main isotopes of chlorine.(Solved)
Identify the two main isotopes of chlorine.
Date posted: August 16, 2019. Answers (1)
- Study the set up below and answer the questions that follow.(Solved)
Study the set up below and answer the questions that follow.
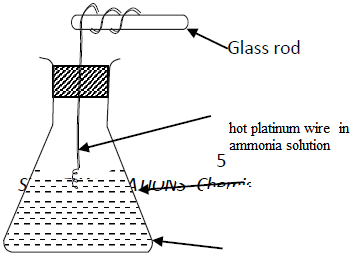
(a) State and explain the observations made in the conical.
(b) Write an equation for the reaction that takes place in the conical flask.
Date posted: August 16, 2019. Answers (1)
- Using an energy cycle diagram, determine the enthalpy formation of ethanol, given that:(Solved)
Using an energy cycle diagram, determine the enthalpy formation of ethanol, given that:
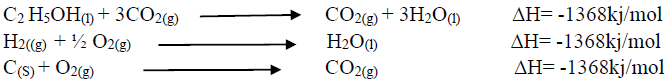
Date posted: August 16, 2019. Answers (1)
- During the manufacture of sodium carbonate by solvay process,there is a by-product produced which is not recycled.(Solved)
During the manufacture of sodium carbonate by solvay process,there is a by-product produced which is not recycled.
(a) Write the equation for the formation of this by-product.
(b) State one of the uses of the product (a) above.
(c) State one use of the sodium carbonate formed.
Date posted: August 16, 2019. Answers (1)
- With explanation, state whether the following equation has undergone an oxidation or reduction process.(Solved)
With explanation, state whether the following equation has undergone an oxidation or reduction process.

Date posted: August 16, 2019. Answers (1)
- A piece of cheese contains proteins which are made up of amino acid as shown.(Solved)
A piece of cheese contains proteins which are made up of amino acid as shown.

(a) Name the type of polymerization involved in protein formation.
(b) Show the structural formula for the polymer formed when the amino acids polymerise.
Date posted: August 16, 2019. Answers (1)
- Using dots and crosses, show the type of bonding in Calcium Chloride(Solved)
Using dots and crosses, show the type of bonding in Calcium Chloride
Date posted: August 16, 2019. Answers (1)
- A student accidentally mixed three solids: lead (II) sulphate, copper (II) oxide and Iron (III) chloride. Describe how you would obtain a solid lead (II)...(Solved)
A student accidentally mixed three solids: lead (II) sulphate, copper (II) oxide and Iron (III) chloride. Describe how you would obtain a solid lead (II) sulphate from the mixture.
Date posted: August 16, 2019. Answers (1)
- When a few drops of sodium hydroxide solution were added to a colorless test solution, a white precipitate was formed. When excess sodium hydroxide solution...(Solved)
When a few drops of sodium hydroxide solution were added to a colorless test solution, a white precipitate was formed. When excess sodium hydroxide solution was added, the precipitate dissolved. Similar results were obtained with the test solution when aqueous ammonia was used.
(a) Identify the cation present in the test solution.
(b) Identify the precipitate formed with the few drops of sodium hydroxide solution were added
(c) Write a balanced ionic equation for the reaction between the precipitate and excess ammonia solution.
Date posted: August 16, 2019. Answers (1)
- Explain the effect of hydrogen chloride on blue and red litmus papers when it is dissolved in water and in methylbenzene.(Solved)
Explain the effect of hydrogen chloride on blue and red litmus papers when it is dissolved in water and in methylbenzene.
Date posted: August 16, 2019. Answers (1)
- Consider the following equilibrium reaction.(Solved)
Consider the following equilibrium reaction.

Identify the acid and base in the forward reaction. Explain your answer
Date posted: August 16, 2019. Answers (1)