(i) +2.87V
(ii) T2(g) Because it has a zero electrode potential for a standard electrode.
maurice.mutuku answered the question on August 19, 2019 at 08:02
- A volatile liquid N is a compound made of carbon, hydrogen and chlorine 0.40 moles of N contain 9.6g carbon, 1.6g hydrogen and 28.4g chlorine....(Solved)
A volatile liquid N is a compound made of carbon, hydrogen and chlorine 0.40 moles of N contain 9.6g carbon, 1.6g hydrogen and 28.4g chlorine. Determine: (C=12, H=1,Cl=35.5)
i). the relative molecular mass of N
ii). The molecular formula of N
iii). The systematic name of N
Date posted: August 19, 2019. Answers (1)
- Study the flow chart below and answer the questions that follow.(Solved)
Study the flow chart below and answer the questions that follow.
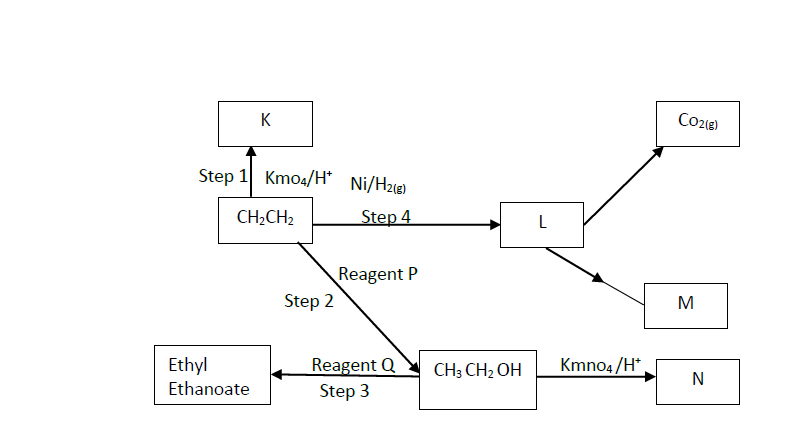
i). Name the following organic compounds.
K
N
ii). Name the process in steps
2
4
iii). Identify the following reagents
P
Q
iv). Write an equation for the reaction between CH3CH2CH2OH and sodium.
Date posted: August 19, 2019. Answers (1)
- Name the following organic compounds.(Solved)
Name the following organic compounds.
i). CH3CH2CH2OH
ii). CH3CH2COOH
iii). CH3CH2COOC2H5
Date posted: August 19, 2019. Answers (1)
- Study the flow chart below and answer the questions that follow.(Solved)
Study the flow chart below and answer the questions that follow.
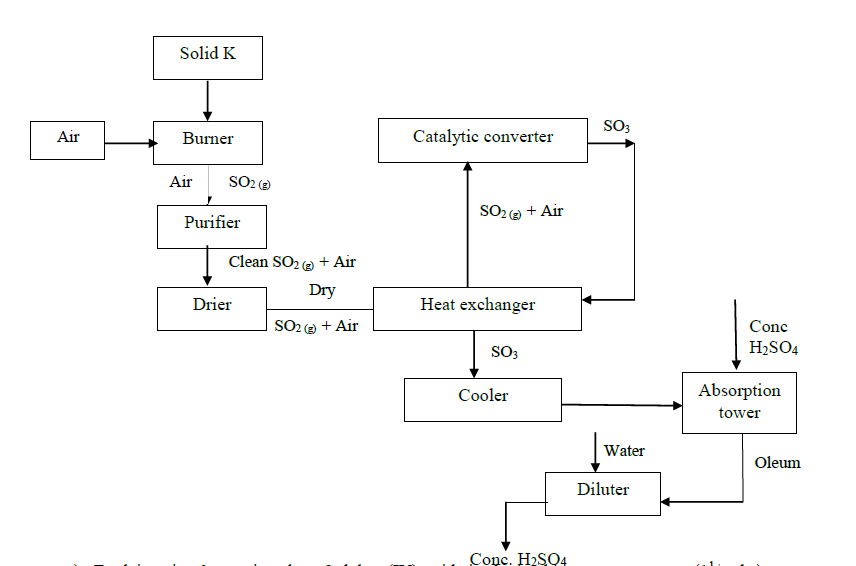
a) Explain using 3 equations how Sulphur (IV) oxide is obtained in contact process.
b) State three identities of solid K.
c) Name two impurities present in the gaseous mixture and suggest how they can be eliminated.
d) Identify the catalyst used in the catalytic converter and state two reasons why it is preferred.
e) Explain why Sulphur (VI) oxide is not absorbed directly into water.
f) Write balanced chemical equations for the reactions taking place at:
i). The catalytic converter
ii). Absorption tower.
iii). Diluter
Date posted: August 19, 2019. Answers (1)
- The grid below shows a part of the periodic table. Study it and answer the questions that follow.The letters are not the actual symbols of...(Solved)
The grid below shows a part of the periodic table. Study it and answer the questions that follow.The letters are not the actual symbols of the elements.
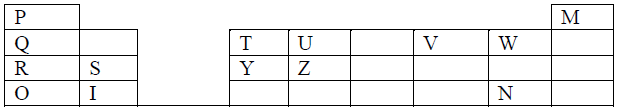
(a) Element P is in group 1 and another group in the periodic table. Identify the other group and give a reason for your answer.
(b) Comment on the reactivity of the group in which element P belongs.
(c) Compare the melting points of elements R and Y.
(d) Compare the electricity conductivity of elements R and Y
(e) Explain the difference in melting points between the oxide of S and that of U
(f) Write an equation for the reaction between elements R and V.
(g) Which of the above elements would form;
(i) . A monovalent anion
(ii). A divalent cation
(h) Explain why element M is placed in group VIII
Date posted: August 19, 2019. Answers (1)
- Draw a well labelled diagram to show the penetrating power of the three types of nuclear radiations.(Solved)
(a) Draw a well labelled diagram to show the penetrating power of the three types of nuclear radiations.
(b) . Which radiation causes more harm to human cells? Explain.
Date posted: August 19, 2019. Answers (1)
- Identify the following substances.(Solved)
Identify the following substances.
(a) (i) 
(ii) 
(b)Which of the above substances is suitable for use with hard water? Explain.
Date posted: August 19, 2019. Answers (1)
- Alkanoic acids have a higher boiling point than the corresponding alkanols.(Solved)
Alkanoic acids have a higher boiling point than the corresponding alkanols. Explain this
Date posted: August 19, 2019. Answers (1)
- Write an equation for the reaction when copper pyrite is roasted in air.(Solved)
Write an equation for the reaction when copper pyrite is roasted in air.
Date posted: August 19, 2019. Answers (1)
- Use the cell representation below to answer the question that follows.(Solved)
Use the cell representation below to answer the question that follows.

Date posted: August 16, 2019. Answers (1)
- Consider the following equilibrium reaction.(Solved)
Consider the following equilibrium reaction.
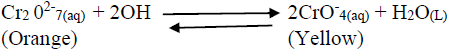
State and explain what would be observed when dilute hydrochloric acid is added into the equilibrium mixture.
Date posted: August 16, 2019. Answers (1)
- 28g of a saturated solution of a salt at 250 C yielded 10g of solid when evaporated to dryness. Calculate the solubility of the salt...(Solved)
28g of a saturated solution of a salt at 250 C yielded 10g of solid when evaporated to dryness. Calculate the solubility of the salt at 250 C
Date posted: August 16, 2019. Answers (1)
- Study the flow diagram below answer the questions that follow.(Solved)
Study the flow diagram below answer the questions that follow.

(a) Name liquid L
(b) Write an ionic equation for the formation of Solid X
Date posted: August 16, 2019. Answers (1)
- Using equations, distinguish between the bleaching action of chlorine and Sulphur (IV) oxide.(Solved)
Using equations, distinguish between the bleaching action of chlorine and Sulphur (IV) oxide.
Date posted: August 16, 2019. Answers (1)
- Identify the two main isotopes of chlorine.(Solved)
Identify the two main isotopes of chlorine.
Date posted: August 16, 2019. Answers (1)
- Study the set up below and answer the questions that follow.(Solved)
Study the set up below and answer the questions that follow.
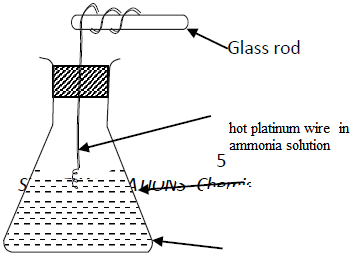
(a) State and explain the observations made in the conical.
(b) Write an equation for the reaction that takes place in the conical flask.
Date posted: August 16, 2019. Answers (1)
- Using an energy cycle diagram, determine the enthalpy formation of ethanol, given that:(Solved)
Using an energy cycle diagram, determine the enthalpy formation of ethanol, given that:
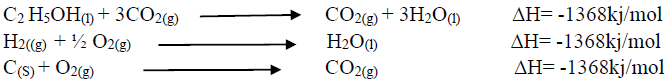
Date posted: August 16, 2019. Answers (1)
- During the manufacture of sodium carbonate by solvay process,there is a by-product produced which is not recycled.(Solved)
During the manufacture of sodium carbonate by solvay process,there is a by-product produced which is not recycled.
(a) Write the equation for the formation of this by-product.
(b) State one of the uses of the product (a) above.
(c) State one use of the sodium carbonate formed.
Date posted: August 16, 2019. Answers (1)
- With explanation, state whether the following equation has undergone an oxidation or reduction process.(Solved)
With explanation, state whether the following equation has undergone an oxidation or reduction process.

Date posted: August 16, 2019. Answers (1)
- A piece of cheese contains proteins which are made up of amino acid as shown.(Solved)
A piece of cheese contains proteins which are made up of amino acid as shown.

(a) Name the type of polymerization involved in protein formation.
(b) Show the structural formula for the polymer formed when the amino acids polymerise.
Date posted: August 16, 2019. Answers (1)