- The figure below shows a toy car moving in a circular rail in a vertical plane. The mass of the toy car is 300g and...(Solved)
The figure below shows a toy car moving in a circular rail in a vertical plane. The mass of the toy car is 300g and the radius of the rail is 2m.
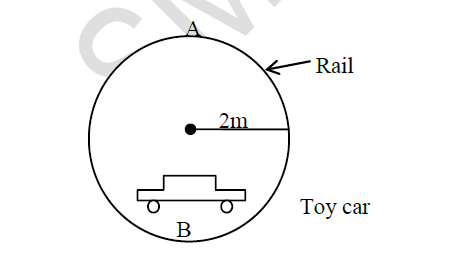
Determine:
i) Minimum velocity at which the toy passes point A
ii) If the toy was tied by a string at the centre of the rail path, following the same circular path, when whirled at which position (A or B) would the string experience maximum tension. Explain your answer.
iii) If the toy moves with a velocity of 5m/s as it passes point B, find the angular velocity at this point.
iv) State two applications of uniform circular motion
Date posted: August 22, 2019. Answers (1)
- The diagram in the figure below represents a wheel and axle used as a machine, whose efficiency is 80% to raise 400N of building materials....(Solved)
The diagram in the figure below represents a wheel and axle used as a machine, whose efficiency is 80% to raise 400N of building materials. The wheel and axle have diameters of 75cm and 15cm respectively.

(i) Mark on the diagram the correct position and direction of the load to be lifted.
(ii) Name the principle on which this machine works.
(iii) Calculate the effort needed to raise the load.
(iv) The machine is operated manually and raises the load to a height of 5m in 20 seconds. Calculate the power developed by the operator
Date posted: August 22, 2019. Answers (1)
- A simple hydrometer is set up with a test –tube of mass 10g and length 12cm with a flat base and partially filled with lead...(Solved)
A simple hydrometer is set up with a test –tube of mass 10g and length 12cm with a flat base and partially filled with lead shots. The test – tube has a uniform cross – sectional area of 2.0cm2 and 10cm of its length is under water as shown in the figure below.
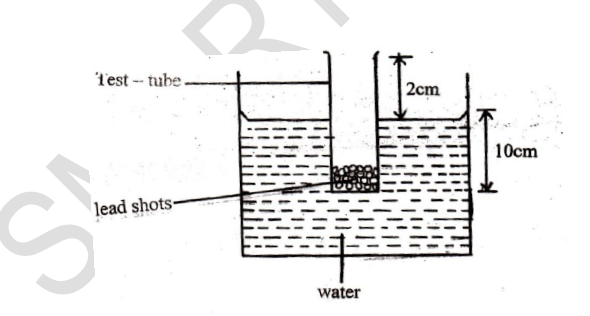
(i) Determine the mass of lead shots in the test – tube (Take density of water = 1000kgm-3)
(ii) Calculate the mass of the lead shots to be added if the test – tube has to displace an equal volume of a liquid of density 1.25gcm-3.
(iii) What is the function of the lead shots?
Date posted: August 22, 2019. Answers (1)
- Hooke’s law is written mathematically as F=Ke. Give three factors that letter K depend on.(Solved)
Hooke’s law is written mathematically as F=Ke. Give three factors that letter K depend on.
Date posted: August 22, 2019. Answers (1)
- A light spring that obeys Hooke’s law was attached to a fixed support. When a load of 10N was hung on it, the length of the...(Solved)
A light spring that obeys Hooke’s law was attached to a fixed support. When a load of 10N was hung on it, the length of the spring was 320mm and when a 20N load was hung on it, the length became 400mm. Determine:
(i) The length of the spring when it has no load on it
(ii) The length of the spring when it has a load of 16N.
Date posted: August 22, 2019. Answers (1)
- The figure below shows two graphs for two different springs A and B(Solved)
The figure below shows two graphs for two different springs A and B
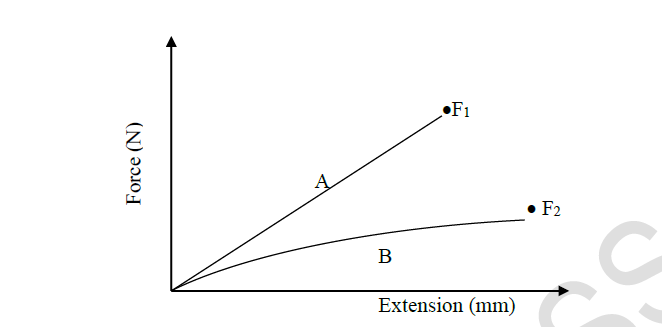
F1 and F2 are points at which the springs break.
Determine and explain which of the two springs:
(i) Obeys Hooke’s law
(ii) Is stronger
Date posted: August 22, 2019. Answers (1)
- The diagram below shows a pendulum bob swinging freely to and from.(Solved)
The diagram below shows a pendulum bob swinging freely to and from.
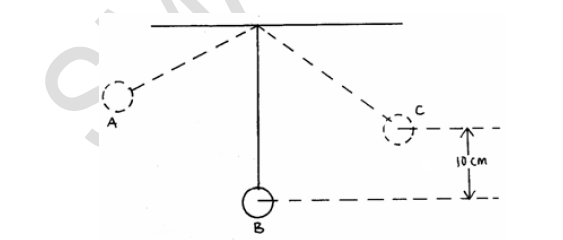
Determine the maximum velocity of the ball at B if the maximum horizontal displacement of the ball is 10cm.
Date posted: August 22, 2019. Answers (1)
- A ball rolls off a platform of height 1.8 m at a horizontal speed of 15m/s. How far off the edge of the platform does...(Solved)
A ball rolls off a platform of height 1.8 m at a horizontal speed of 15m/s. How far off the edge of the platform does it land? (Take g = 10msˉ²)
Date posted: August 22, 2019. Answers (1)
- Explain the difference between the motion of dust particles in Brownian motion and the motion of dust particles due to convectional currents.(Solved)
Explain the difference between the motion of dust particles in Brownian motion and the motion of dust particles due to convectional currents.
Date posted: August 22, 2019. Answers (1)
- The figure I below shows the reading of a vernier calipers used to get the diameter of a cylindrical tin.(Solved)
The figure I below shows the reading of a vernier calipers used to get the diameter of a cylindrical tin.
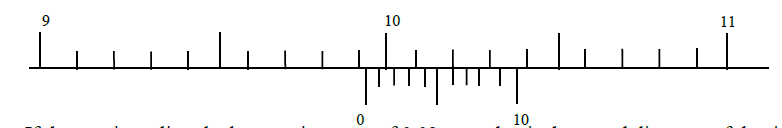
If the vernier caliper had a negative error of 0.02 cm, what is the actual diameter of the tin?
Date posted: August 22, 2019. Answers (1)
- i)Derive an expression for the heat lost by the steam as it condenses to water at temperature T.
ii)Derive an expression for the heat gain in...(Solved)
Steam of mass 3.0g at 100 degress celsius is passed into water of mass 400g at 10 degrees celsius. the final temperature of the mixture is T. The container absorbs negligible heat. (specific latent heat of vaporization of steam = 2260KJ/kg, specific heat capacity of water =4200J Kg-1 K-)
Date posted: August 13, 2019. Answers (1)
- The graph shows variation of extension and stretching force F for a spring which obeys Hooke’s law.
(i) Determine the spring constant in SI units .
(ii)...(Solved)
The graph shows variation of extension and stretching force F for a spring which obeys Hooke’s law.
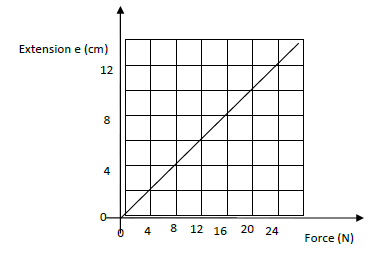
(i) Determine the spring constant in SI units .
(ii) The energy stored when the extension is 20 cm.
Date posted: August 6, 2019. Answers (1)
- The figure below shows two identical metallic cans A and B, filled with water at room temperature. An electric heater is placed at equal distances...(Solved)
The figure below shows two identical metallic cans A and B, filled with water at room temperature. An electric heater is placed at equal distances from A and B.

In the figure below sketch a graph to show the variation of temperature with time for the two surfaces.

Date posted: August 6, 2019. Answers (1)
- A ball bearing of mass 1.5 x 10-4 kg is held between the anvil and spindle of a micrometer screw
gauge as shown in the figure...(Solved)
A ball bearing of mass 1.5 x 10-4 kg is held between the anvil and spindle of a micrometer screw
gauge as shown in the figure below.
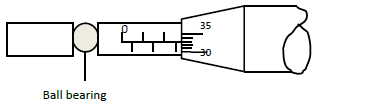
Before the instrument was the zero error was found to be 0.03 mm.
(i) What is the diameter of the ball bearing?
(ii) Find the density of the ball bearing correct to 3 significant figures and in SI units.
Date posted: August 6, 2019. Answers (1)
- Explain why when graduating the upper fixed point of a thermometer; the bulb is put in steam
above boiling water and not boiling water.(Solved)
Explain why when graduating the upper fixed point of a thermometer; the bulb is put in steam
above boiling water and not boiling water.
Date posted: August 6, 2019. Answers (1)
- The figure below shows a soap film formed on a metal ring and a loop of thread inside it.
(i) Explain what will happen when the...(Solved)
The figure below shows a soap film formed on a metal ring and a loop of thread inside it.

(i) Explain what will happen when the film is punctured by a needle at X.
(ii) What is the name given to the force acting on the thread?
Date posted: August 6, 2019. Answers (1)
- In an experiment to determine the relative density of a substance using a density bottle the
following measurements were taken.
- Mass of empty density bottle =...(Solved)
In an experiment to determine the relative density of a substance using a density bottle the
following measurements were taken.
- Mass of empty density bottle = 43.2 g
- Mass of bottle full of water = 66.4 g
- Mass of bottle filled with liquid X = 68.2g
Use the data to determine the density of the liquids.
Date posted: August 6, 2019. Answers (1)
- The figure below shows the path of ray of yellow light through a glass prism. The speed of
yellow light in the prism is 1.8 x...(Solved)
The figure below shows the path of ray of yellow light through a glass prism. The speed of
yellow light in the prism is 1.8 x 108m/s.

(i) Determine the refractive index of the prism material (Speed of light in vacuum, C = 3.0 x 108m/s)
(ii) Show on the same diagram, the critical angle C and hence determine its value.
Date posted: August 6, 2019. Answers (1)
- The diagram below shows a narrow beam of white light onto a glass prism.
(i) What is the name of the phenomenon represented in the diagram?...(Solved)
The diagram below shows a narrow beam of white light onto a glass prism.
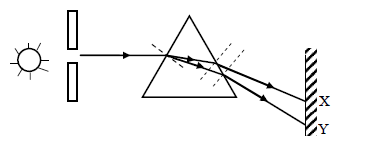
(i) What is the name of the phenomenon represented in the diagram?
(ii) Name the color at X and Y
Date posted: August 6, 2019. Answers (1)
- A 5µF capacitor is charged to a potential difference of 200V and isolated. It is then connected
to a 10µF capacitor.Find
(i) The resultant potential difference across...(Solved)
A 5μF capacitor is charged to a potential difference of 200V and isolated. It is then connected
to a 10μF capacitor.Find
(i) The resultant potential difference across the combination
(ii) Energy stored before connection
(iii) Total energy in the capacitors after connection
Date posted: August 6, 2019. Answers (1)