- In order to prepare hydrogen gas in the laboratory a student set-up the apparatus shown in the diagram below. Study it and answer the questions...(Solved)
In order to prepare hydrogen gas in the laboratory a student set-up the apparatus shown in the diagram below. Study it and answer the questions that follow.
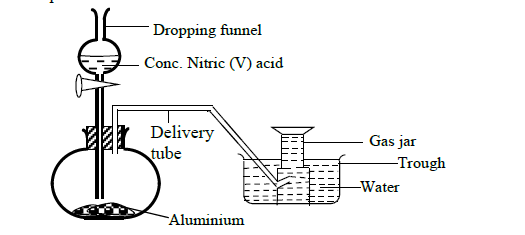
(a) Suggest why the student did not collect hydrogen gas.
(b) In a separate experiment the student reacted iron and hydrochloric acid to prepare hydrogen gas.
(i) Write an ionic equation for the reaction.
(ii) The hydrogen gas produced was found to have a foul smell. Suggest an explanation for this.
Date posted: September 3, 2019. Answers (1)
- Study the structure below.
C3H7COOC2H5
(a) Name the compound
(b) Name the compounds used to prepare the above compound.
(c) What is the identifying physical property of...(Solved)
Study the structure below.
C3H7COOC2H5
(a) Name the compound
(b) Name the compounds used to prepare the above compound.
(c) What is the identifying physical property of the above compound?
Date posted: September 3, 2019. Answers (1)
- Study the flow chart below and answer the questions that follow.(Solved)
Study the flow chart below and answer the questions that follow.
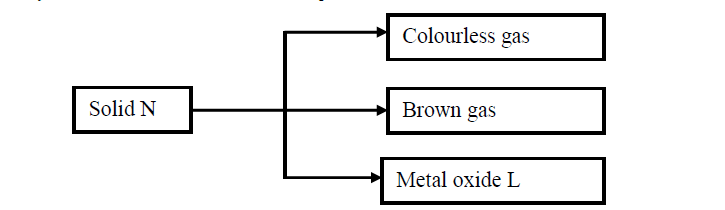
(a) Write the formula of the anion present in solid N.
(b) Solid N in the flow chart above burns in air with a red flame. Identify the
(i) Cation present in solid N
(ii) Metal oxide L
Date posted: September 3, 2019. Answers (1)
- Study the table below and answer the questions that follow.(Solved)
Study the table below and answer the questions that follow.
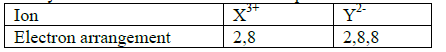
(a) Write the electronic arrangement of elements. X and Y
(b) Write the formula of the compound that would be formed between X and Y.
Date posted: September 3, 2019. Answers (1)
- Element R – 238 decays in series forming different nuclides as shown below.(Solved)
Element R – 238 decays in series forming different nuclides as shown below.
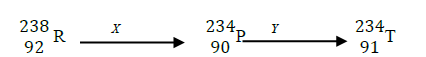
(i) Identify the type of decay X and Y
(ii) Give one use of radioactive isotopes in medicine
Date posted: September 3, 2019. Answers (1)
- Identify the particles which enable the following substances to conduct electricity.
(i) Aluminium metal
(ii) Molten lead (II) bromide(Solved)
Identify the particles which enable the following substances to conduct electricity.
(i) Aluminium metal
(ii) Molten lead (II) bromide
Date posted: September 3, 2019. Answers (1)
- The table below shows PH values of substances A, B, C and D. Study it and answer the questions that follow.(Solved)
The table below shows PH values of substances A, B, C and D. Study it and answer the questions that follow.

(a) Which substance is likely to be pure water
(b) Which solution contains the lowest concentration of hydrogen ions?
(c) In the equation below, identify the reagent that acts as a base. Give a reason for your answer.

Date posted: September 3, 2019. Answers (1)
- Give the I.U.P.A.C name of the oxide of nitrogen that:-
(i) Relights a glowing splint
(ii) Forms brown complex compound with acidified Iron (II) sulphate solution...(Solved)
Give the I.U.P.A.C name of the oxide of nitrogen that:-
(i) Relights a glowing splint
(ii) Forms brown complex compound with acidified Iron (II) sulphate solution
(iii) Reacts with water to form nitric (V) acid
Date posted: September 3, 2019. Answers (1)
- The following represents a Bunsen Burner flame.(Solved)
The following represents a Bunsen Burner flame.
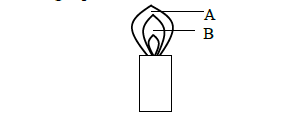
(a) Name the parts of the flame labeled A and B.
(b) Identify the hottest part of the flame. Give a reason.
Date posted: September 3, 2019. Answers (1)
- Draw a well labelled diagram to show how you would prepare and collect dry chlorine gas in the laboratory.(Solved)
(a) Draw a well labelled diagram to show how you would prepare and collect dry chlorine gas in the laboratory.
(b) A part from the reagents used in (a) above, name two other sets of reagents that can be used to prepare chlorine gas.
(c) Describe how you would test for chloride ions in a solid sample suspected to contain the ions in the laboratory.
Date posted: August 19, 2019. Answers (1)
- How many kilograms of Iron could be obtained from 240kg of Iron (III) Oxide. (Fe=56.0 O= 16.00)(Solved)
How many kilograms of Iron could be obtained from 240kg of Iron (III) Oxide. (Fe=56.0 O= 16.00)
Date posted: August 19, 2019. Answers (1)
- The diagram below shows a blast furnace used for extraction of iron(Solved)
The diagram below shows a blast furnace used for extraction of iron

(a) State how high temperatures in region B is maintained.
(b) Name the reducing agent in the process above.
(c) Identify;
(i) Y
(ii) Z
(d) Explain why it is desirable for compound Y to stay on top of Z.
(e) Write an equation for formation of Z.
Date posted: August 19, 2019. Answers (1)
- Explain the following observation:(Solved)
Explain the following observation:
The melting and boiling points of alkanoic acids increases with increase in the number of carbon atoms in the molecules.
Date posted: August 19, 2019. Answers (1)
- Study the standard electrode potentials for the elements given below and answer the questions that follow.(Solved)
Study the standard electrode potentials for the elements given below and answer the questions that follow.
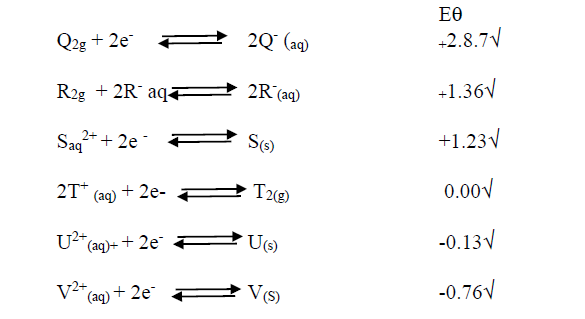
i). What is the 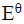 value of the weakest reducing agent?
value of the weakest reducing agent?
ii). Which element is likely to be hydrogen? Give a reason for your answer.
Date posted: August 19, 2019. Answers (1)
- A volatile liquid N is a compound made of carbon, hydrogen and chlorine 0.40 moles of N contain 9.6g carbon, 1.6g hydrogen and 28.4g chlorine....(Solved)
A volatile liquid N is a compound made of carbon, hydrogen and chlorine 0.40 moles of N contain 9.6g carbon, 1.6g hydrogen and 28.4g chlorine. Determine: (C=12, H=1,Cl=35.5)
i). the relative molecular mass of N
ii). The molecular formula of N
iii). The systematic name of N
Date posted: August 19, 2019. Answers (1)
- Study the flow chart below and answer the questions that follow.(Solved)
Study the flow chart below and answer the questions that follow.
i). Name the following organic compounds.
K
N
ii). Name the process in steps
2
4
iii). Identify the following reagents
P
Q
iv). Write an equation for the reaction between CH3CH2CH2OH and sodium.
Date posted: August 19, 2019. Answers (1)
- Name the following organic compounds.(Solved)
Name the following organic compounds.
i). CH3CH2CH2OH
ii). CH3CH2COOH
iii). CH3CH2COOC2H5
Date posted: August 19, 2019. Answers (1)
- Study the flow chart below and answer the questions that follow.(Solved)
Study the flow chart below and answer the questions that follow.
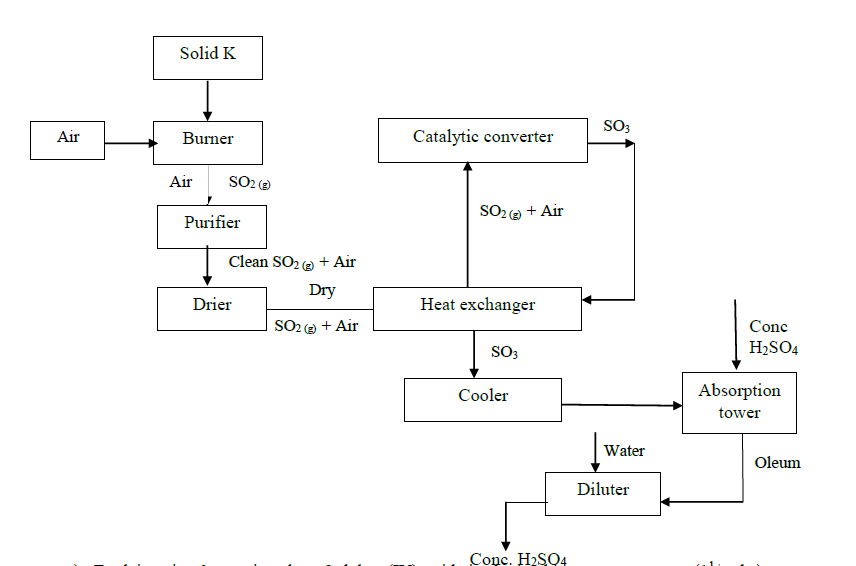
a) Explain using 3 equations how Sulphur (IV) oxide is obtained in contact process.
b) State three identities of solid K.
c) Name two impurities present in the gaseous mixture and suggest how they can be eliminated.
d) Identify the catalyst used in the catalytic converter and state two reasons why it is preferred.
e) Explain why Sulphur (VI) oxide is not absorbed directly into water.
f) Write balanced chemical equations for the reactions taking place at:
i). The catalytic converter
ii). Absorption tower.
iii). Diluter
Date posted: August 19, 2019. Answers (1)
- The grid below shows a part of the periodic table. Study it and answer the questions that follow.The letters are not the actual symbols of...(Solved)
The grid below shows a part of the periodic table. Study it and answer the questions that follow.The letters are not the actual symbols of the elements.
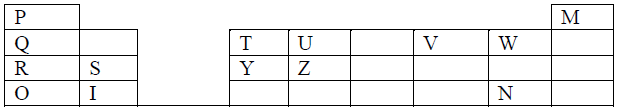
(a) Element P is in group 1 and another group in the periodic table. Identify the other group and give a reason for your answer.
(b) Comment on the reactivity of the group in which element P belongs.
(c) Compare the melting points of elements R and Y.
(d) Compare the electricity conductivity of elements R and Y
(e) Explain the difference in melting points between the oxide of S and that of U
(f) Write an equation for the reaction between elements R and V.
(g) Which of the above elements would form;
(i) . A monovalent anion
(ii). A divalent cation
(h) Explain why element M is placed in group VIII
Date posted: August 19, 2019. Answers (1)
- Draw a well labelled diagram to show the penetrating power of the three types of nuclear radiations.(Solved)
(a) Draw a well labelled diagram to show the penetrating power of the three types of nuclear radiations.
(b) . Which radiation causes more harm to human cells? Explain.
Date posted: August 19, 2019. Answers (1)