i) Forward reaction favoured since reaction is endothermic ; more HI produced.
ii) Reactant side 2V products side 2V volumes are the same therefore pressure has no effect.
maurice.mutuku answered the question on September 3, 2019 at 08:37
-
The table below shows elements in the halogen group of the periodic table. Study the table and answer the questions that follow.
(Solved)
The table below shows elements in the halogen group of the periodic table. Study the table and answer the questions that follow.

(i) Name the element likely to be a solid at room temperature. Explain
(ii) Explain why the melting point increases from fluorine to iodine.
Date posted:
September 3, 2019
.
Answers (1)
-
Two gases A and B have relative densities of 1.98 and 2.90 respectively. They diffuse under the same conditions.
(i) Compare their rates of diffusion
(ii) Determine...
(Solved)
Two gases A and B have relative densities of 1.98 and 2.90 respectively. They diffuse under the same conditions.
(i) Compare their rates of diffusion
(ii) Determine the relative molecular mass of A, given that the relative molecular mass of B is 64.
Date posted:
September 3, 2019
.
Answers (1)
-
When excess chlorine is bubbled through cold dilute sodium hydroxide solution, the resulting solution is a bleaching agent.
(a) Write a chemical equation for the reaction...
(Solved)
When excess chlorine is bubbled through cold dilute sodium hydroxide solution, the resulting solution is a bleaching agent.
(a) Write a chemical equation for the reaction that produces the bleaching agent.
(b) Name the bleaching compound and show how it bleaches using an equation.
Date posted:
September 3, 2019
.
Answers (1)
-
In order to prepare hydrogen gas in the laboratory a student set-up the apparatus shown in the diagram below. Study it and answer the questions...
(Solved)
In order to prepare hydrogen gas in the laboratory a student set-up the apparatus shown in the diagram below. Study it and answer the questions that follow.
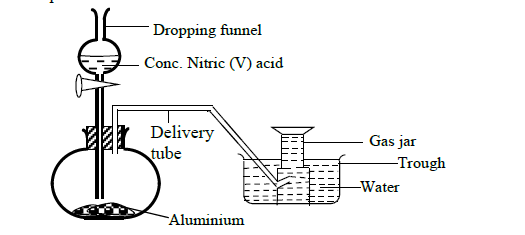
(a) Suggest why the student did not collect hydrogen gas.
(b) In a separate experiment the student reacted iron and hydrochloric acid to prepare hydrogen gas.
(i) Write an ionic equation for the reaction.
(ii) The hydrogen gas produced was found to have a foul smell. Suggest an explanation for this.
Date posted:
September 3, 2019
.
Answers (1)
-
Study the flow chart below and answer the questions that follow.
(Solved)
Study the flow chart below and answer the questions that follow.
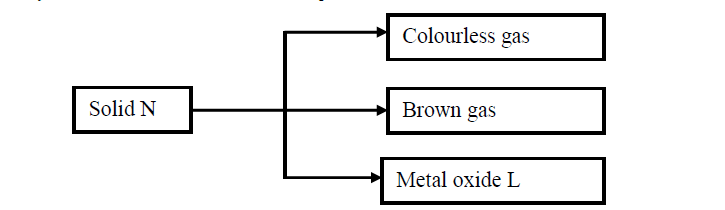
(a) Write the formula of the anion present in solid N.
(b) Solid N in the flow chart above burns in air with a red flame. Identify the
(i) Cation present in solid N
(ii) Metal oxide L
Date posted:
September 3, 2019
.
Answers (1)
-
Give the I.U.P.A.C name of the oxide of nitrogen that:-
(i) Relights a glowing splint
(ii) Forms brown complex compound with acidified Iron (II) sulphate solution...
(Solved)
Give the I.U.P.A.C name of the oxide of nitrogen that:-
(i) Relights a glowing splint
(ii) Forms brown complex compound with acidified Iron (II) sulphate solution
(iii) Reacts with water to form nitric (V) acid
Date posted:
September 3, 2019
.
Answers (1)
-
Draw a well labelled diagram to show how you would prepare and collect dry chlorine gas in the laboratory.
(Solved)
(a) Draw a well labelled diagram to show how you would prepare and collect dry chlorine gas in the laboratory.
(b) A part from the reagents used in (a) above, name two other sets of reagents that can be used to prepare chlorine gas.
(c) Describe how you would test for chloride ions in a solid sample suspected to contain the ions in the laboratory.
Date posted:
August 19, 2019
.
Answers (1)
-
The diagram below shows a blast furnace used for extraction of iron
(Solved)
The diagram below shows a blast furnace used for extraction of iron

(a) State how high temperatures in region B is maintained.
(b) Name the reducing agent in the process above.
(c) Identify;
(i) Y
(ii) Z
(d) Explain why it is desirable for compound Y to stay on top of Z.
(e) Write an equation for formation of Z.
Date posted:
August 19, 2019
.
Answers (1)
-
Study the flow chart below and answer the questions that follow.
(Solved)
Study the flow chart below and answer the questions that follow.
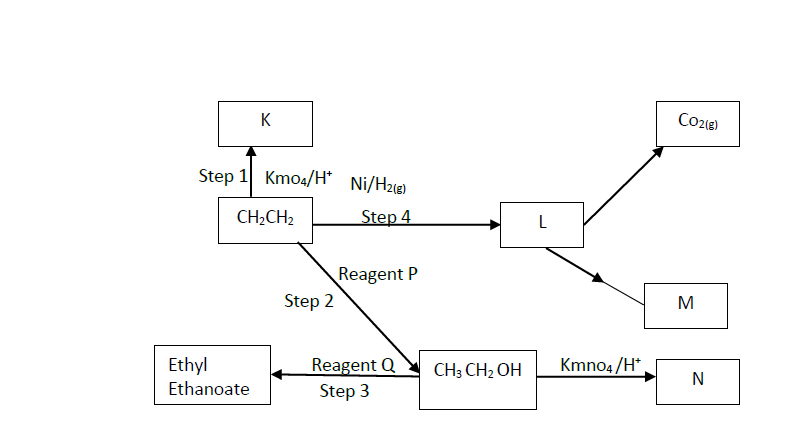
i). Name the following organic compounds.
K
N
ii). Name the process in steps
2
4
iii). Identify the following reagents
P
Q
iv). Write an equation for the reaction between CH3CH2CH2OH and sodium.
Date posted:
August 19, 2019
.
Answers (1)
-
Study the flow chart below and answer the questions that follow.
(Solved)
Study the flow chart below and answer the questions that follow.
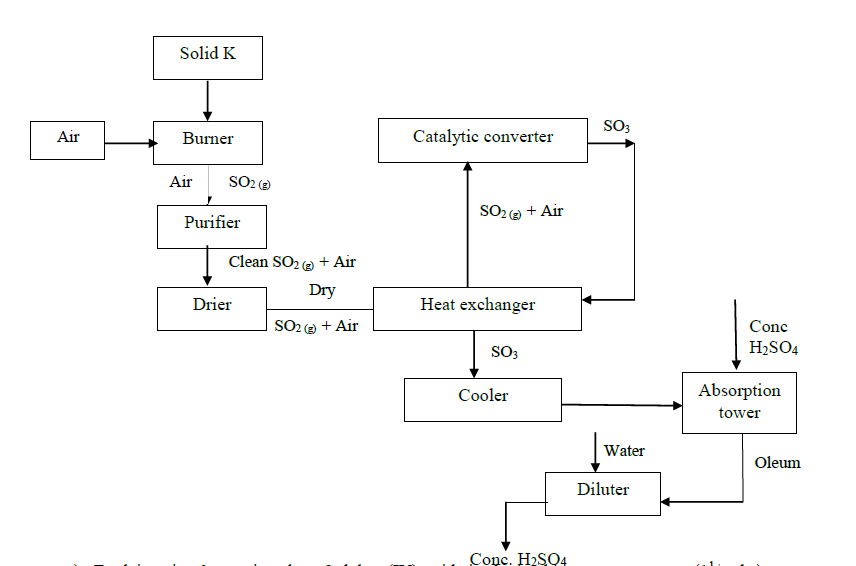
a) Explain using 3 equations how Sulphur (IV) oxide is obtained in contact process.
b) State three identities of solid K.
c) Name two impurities present in the gaseous mixture and suggest how they can be eliminated.
d) Identify the catalyst used in the catalytic converter and state two reasons why it is preferred.
e) Explain why Sulphur (VI) oxide is not absorbed directly into water.
f) Write balanced chemical equations for the reactions taking place at:
i). The catalytic converter
ii). Absorption tower.
iii). Diluter
Date posted:
August 19, 2019
.
Answers (1)
-
Using equations, distinguish between the bleaching action of chlorine and Sulphur (IV) oxide.
(Solved)
Using equations, distinguish between the bleaching action of chlorine and Sulphur (IV) oxide.
Date posted:
August 16, 2019
.
Answers (1)
-
Using an energy cycle diagram, determine the enthalpy formation of ethanol, given that:
(Solved)
Using an energy cycle diagram, determine the enthalpy formation of ethanol, given that:
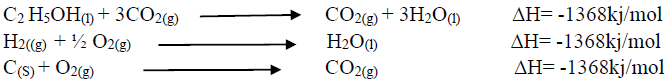
Date posted:
August 16, 2019
.
Answers (1)
-
During the manufacture of sodium carbonate by solvay process,there is a by-product produced which is not recycled.
(Solved)
During the manufacture of sodium carbonate by solvay process,there is a by-product produced which is not recycled.
(a) Write the equation for the formation of this by-product.
(b) State one of the uses of the product (a) above.
(c) State one use of the sodium carbonate formed.
Date posted:
August 16, 2019
.
Answers (1)
-
Using dots and crosses, show the type of bonding in Calcium Chloride
(Solved)
Using dots and crosses, show the type of bonding in Calcium Chloride
Date posted:
August 16, 2019
.
Answers (1)
-
Explain the effect of hydrogen chloride on blue and red litmus papers when it is dissolved in water and in methylbenzene.
(Solved)
Explain the effect of hydrogen chloride on blue and red litmus papers when it is dissolved in water and in methylbenzene.
Date posted:
August 16, 2019
.
Answers (1)
-
The diagram below represents a mercury cell chlor-alkali process that can be used in the industrial manufacture of sodium hydroxide. Study it and answer the...
(Solved)
The diagram below represents a mercury cell chlor-alkali process that can be used in the industrial manufacture of sodium hydroxide. Study it and answer the questions that follow.
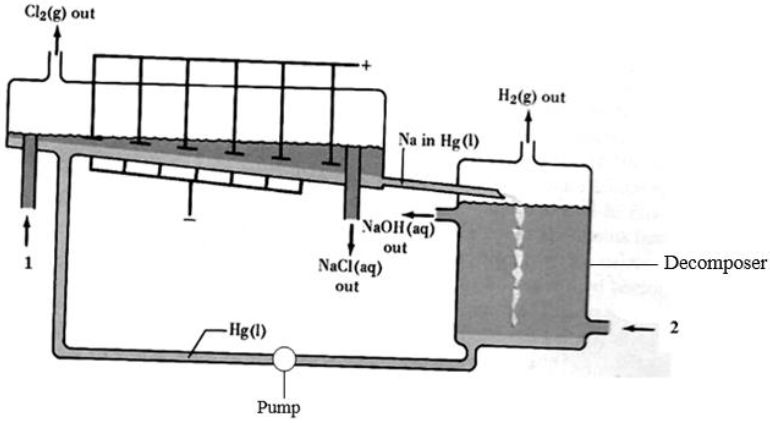
(a) (i) Name the raw materials introduced at 1 and 2.
(ii) Identify a substance that can be used as anode.
(iii) Write equations for the reactions taking place at:
Cathode
Decomposer
(iv) How is the aqueous sodium hydroxide purified?
Date posted:
August 16, 2019
.
Answers (1)
-
The scheme below shows several reactions starting with propanol. Study the scheme and answer the questions which follow.
(Solved)
The scheme below shows several reactions starting with propanol. Study the scheme and answer the questions which follow.

(a) (i) Name gas L.
(ii) Name and draw the structural formula of compound K.
(iii) What conditions and reagents are necessary to convert M and N?
Reagents
Conditions
(iv) Write chemical equation for the conversion of K to J.
Date posted:
August 16, 2019
.
Answers (1)
-
Given that:
Enthalpy of combustion of Carbon (graphite) = -393kJmol-1
Enthalpy of combustion of carbon (II) oxide = -283 kJmol-1
(Solved)
Given that:
Enthalpy of combustion of Carbon (graphite) = -393kJmol-1
Enthalpy of combustion of carbon (II) oxide = -283 kJmol-1
(a) Write down the thermo-chemical equations for the combustion of:
(i) Carbon (graphite)
(ii) Carbon (II) oxide
(b) Determine the enthalpy of formation of carbon (II) oxide.
(c) Draw an energy level diagram for the processes in (a) and (b) above.
(d) State two properties of Carbon (IV) Oxide that make it suitable for use in fire extinguishers.
(e) When 8.0 g of sulphur is completely burned in oxygen in a calorimeter, the heat evolved raises the temperature of 500 cm3 of water by 35 0C. Calculate the heat of combustion of sulphur.
(The specific heat capacity of water = 
Date posted:
August 16, 2019
.
Answers (1)
-
The table below shows the radioactive decay for a sample of iodine -131.Study it and answer the questions that follow.
(Solved)
The table below shows the radioactive decay for a sample of iodine -131.Study it and answer the questions that follow.

(i) Plot the decay curve of iodine -131 using the data given in the table above.
(ii) Use the graph to determine the half-life of the sample.
(iii) What fraction of the original sample remains after 18 days?
Date posted:
August 16, 2019
.
Answers (1)
-
Study the diagram below and answer the questions that follow.
(Solved)
Study the diagram below and answer the questions that follow.

(i) State the observation made in the lime water. Explain.
(ii) How can the identity of substance K be demonstrated?
Date posted:
August 16, 2019
.
Answers (1)