- Production of hydrogen iodide can be demonstrated by the equation below(Solved)
Production of hydrogen iodide can be demonstrated by the equation below
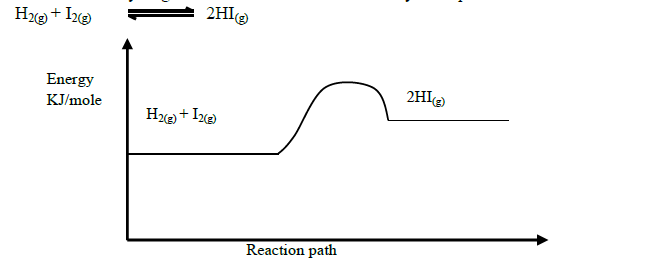
Explain how the following would affect the yield of hydrogen iodide.
(i) Increase in temperature
(ii) Decrease in pressure
Date posted: September 3, 2019. Answers (1)
- Study the following flow chart and answer the questions that follow.(Solved)
Study the following flow chart and answer the questions that follow.

(i) Name:
I. Alcohol R
II. Compound S
(ii) Name process T
Date posted: September 3, 2019. Answers (1)
- The table below shows elements in the halogen group of the periodic table. Study the table and answer the questions that follow.(Solved)
The table below shows elements in the halogen group of the periodic table. Study the table and answer the questions that follow.

(i) Name the element likely to be a solid at room temperature. Explain
(ii) Explain why the melting point increases from fluorine to iodine.
Date posted: September 3, 2019. Answers (1)
- Two gases A and B have relative densities of 1.98 and 2.90 respectively. They diffuse under the same conditions.
(i) Compare their rates of diffusion
(ii) Determine...(Solved)
Two gases A and B have relative densities of 1.98 and 2.90 respectively. They diffuse under the same conditions.
(i) Compare their rates of diffusion
(ii) Determine the relative molecular mass of A, given that the relative molecular mass of B is 64.
Date posted: September 3, 2019. Answers (1)
- During extraction of zinc metal, the ore is subjected to froth floatation. Give a reason why this process is necessary.(Solved)
During extraction of zinc metal, the ore is subjected to froth floatation. Give a reason why this process is necessary.
Date posted: September 3, 2019. Answers (1)
- When excess chlorine is bubbled through cold dilute sodium hydroxide solution, the resulting solution is a bleaching agent.
(a) Write a chemical equation for the reaction...(Solved)
When excess chlorine is bubbled through cold dilute sodium hydroxide solution, the resulting solution is a bleaching agent.
(a) Write a chemical equation for the reaction that produces the bleaching agent.
(b) Name the bleaching compound and show how it bleaches using an equation.
Date posted: September 3, 2019. Answers (1)
- In an experiment, excess magnesium ribbons were immersed in ethanoic acid and the gas evolved was measured at 10 seconds intervals.
(a) Write an equation for...(Solved)
In an experiment, excess magnesium ribbons were immersed in ethanoic acid and the gas evolved was measured at 10 seconds intervals.
(a) Write an equation for the reaction between Ethanoic acid and magnesium ribbon.
(b) Sketch a curve of volume of gas evolved against time for the above reaction.
(c) On the same axis above sketch the curve that would be obtained if hydrochloric acid was used. Label the curve 1.
Date posted: September 3, 2019. Answers (1)
- In order to prepare hydrogen gas in the laboratory a student set-up the apparatus shown in the diagram below. Study it and answer the questions...(Solved)
In order to prepare hydrogen gas in the laboratory a student set-up the apparatus shown in the diagram below. Study it and answer the questions that follow.
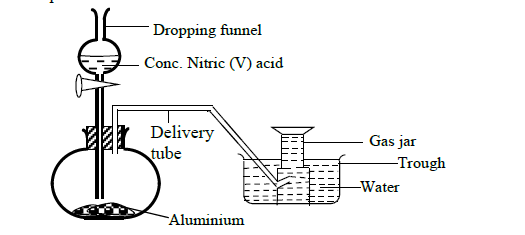
(a) Suggest why the student did not collect hydrogen gas.
(b) In a separate experiment the student reacted iron and hydrochloric acid to prepare hydrogen gas.
(i) Write an ionic equation for the reaction.
(ii) The hydrogen gas produced was found to have a foul smell. Suggest an explanation for this.
Date posted: September 3, 2019. Answers (1)
- Study the structure below.
C3H7COOC2H5
(a) Name the compound
(b) Name the compounds used to prepare the above compound.
(c) What is the identifying physical property of...(Solved)
Study the structure below.
C3H7COOC2H5
(a) Name the compound
(b) Name the compounds used to prepare the above compound.
(c) What is the identifying physical property of the above compound?
Date posted: September 3, 2019. Answers (1)
- Study the flow chart below and answer the questions that follow.(Solved)
Study the flow chart below and answer the questions that follow.
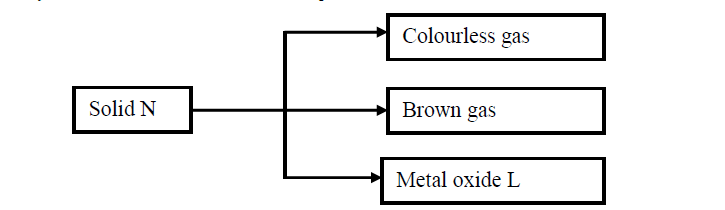
(a) Write the formula of the anion present in solid N.
(b) Solid N in the flow chart above burns in air with a red flame. Identify the
(i) Cation present in solid N
(ii) Metal oxide L
Date posted: September 3, 2019. Answers (1)
- Study the table below and answer the questions that follow.(Solved)
Study the table below and answer the questions that follow.
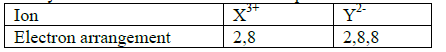
(a) Write the electronic arrangement of elements. X and Y
(b) Write the formula of the compound that would be formed between X and Y.
Date posted: September 3, 2019. Answers (1)
- Element R – 238 decays in series forming different nuclides as shown below.(Solved)
Element R – 238 decays in series forming different nuclides as shown below.
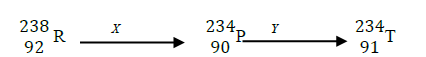
(i) Identify the type of decay X and Y
(ii) Give one use of radioactive isotopes in medicine
Date posted: September 3, 2019. Answers (1)
- Identify the particles which enable the following substances to conduct electricity.
(i) Aluminium metal
(ii) Molten lead (II) bromide(Solved)
Identify the particles which enable the following substances to conduct electricity.
(i) Aluminium metal
(ii) Molten lead (II) bromide
Date posted: September 3, 2019. Answers (1)
- The table below shows PH values of substances A, B, C and D. Study it and answer the questions that follow.(Solved)
The table below shows PH values of substances A, B, C and D. Study it and answer the questions that follow.

(a) Which substance is likely to be pure water
(b) Which solution contains the lowest concentration of hydrogen ions?
(c) In the equation below, identify the reagent that acts as a base. Give a reason for your answer.

Date posted: September 3, 2019. Answers (1)
- Give the I.U.P.A.C name of the oxide of nitrogen that:-
(i) Relights a glowing splint
(ii) Forms brown complex compound with acidified Iron (II) sulphate solution...(Solved)
Give the I.U.P.A.C name of the oxide of nitrogen that:-
(i) Relights a glowing splint
(ii) Forms brown complex compound with acidified Iron (II) sulphate solution
(iii) Reacts with water to form nitric (V) acid
Date posted: September 3, 2019. Answers (1)
- The following represents a Bunsen Burner flame.(Solved)
The following represents a Bunsen Burner flame.
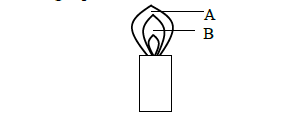
(a) Name the parts of the flame labeled A and B.
(b) Identify the hottest part of the flame. Give a reason.
Date posted: September 3, 2019. Answers (1)
- Draw a well labelled diagram to show how you would prepare and collect dry chlorine gas in the laboratory.(Solved)
(a) Draw a well labelled diagram to show how you would prepare and collect dry chlorine gas in the laboratory.
(b) A part from the reagents used in (a) above, name two other sets of reagents that can be used to prepare chlorine gas.
(c) Describe how you would test for chloride ions in a solid sample suspected to contain the ions in the laboratory.
Date posted: August 19, 2019. Answers (1)
- How many kilograms of Iron could be obtained from 240kg of Iron (III) Oxide. (Fe=56.0 O= 16.00)(Solved)
How many kilograms of Iron could be obtained from 240kg of Iron (III) Oxide. (Fe=56.0 O= 16.00)
Date posted: August 19, 2019. Answers (1)
- The diagram below shows a blast furnace used for extraction of iron(Solved)
The diagram below shows a blast furnace used for extraction of iron

(a) State how high temperatures in region B is maintained.
(b) Name the reducing agent in the process above.
(c) Identify;
(i) Y
(ii) Z
(d) Explain why it is desirable for compound Y to stay on top of Z.
(e) Write an equation for formation of Z.
Date posted: August 19, 2019. Answers (1)
- Explain the following observation:(Solved)
Explain the following observation:
The melting and boiling points of alkanoic acids increases with increase in the number of carbon atoms in the molecules.
Date posted: August 19, 2019. Answers (1)