- Study the standard electrode potentials below and answer the questions that follow. The letters do not represent the actual symbols of the elements.(Solved)
Study the standard electrode potentials below and answer the questions that follow. The letters do not represent the actual symbols of the elements.
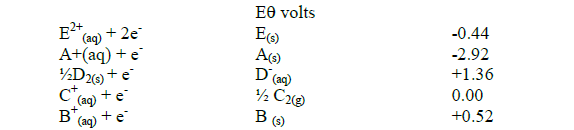
(i) Identify the strongest reducing agent. Give a reason.
(ii) Select two half cells when combined produce the highest potential difference and determine the electromotive force.
(iii) Which element is likely to be hydrogen? Give a reason
(iv) Explain whether the reaction represented below can take place.
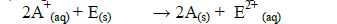
Date posted: September 4, 2019. Answers (1)
- The chart shows the extraction of lead from its ore.(Solved)
The chart shows the extraction of lead from its ore.
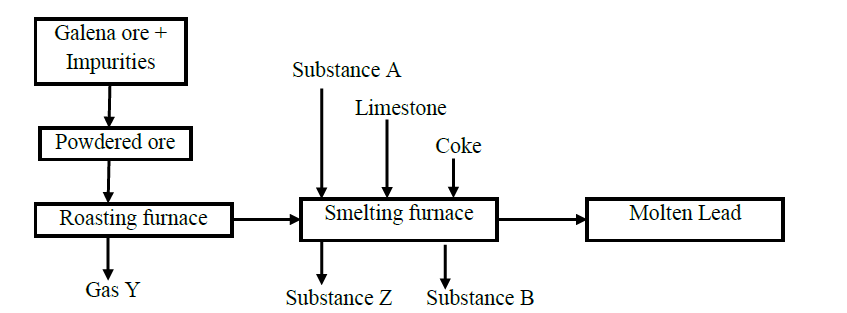
(a) Write the chemical formula of the chief ore.
(b) Name the possible impurities present in the ore.
(c) Why is it necessary for the ore to be converted into powder form?
(d) Identify process X and state it‟s significance
(e) Write equations for the reaction taking place in the
I. Roasting furnace
II. Formation of substance B
It is not advisable to use lead pipes in transporting drinking water.
Explain why.
(f) Identify one of the impurities present in molten lead obtained by the process.
(g) State one use of lead
Date posted: September 4, 2019. Answers (1)
- The flow chart shows some chemical reactions.(Solved)
The flow chart shows some chemical reactions.

(a) Draw the structural formula and names of the following compounds.
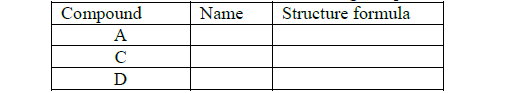
(b) Write the name of the processes that leads to the formation of substances A and F.

(c) Name the type of reaction and conditions required for the formation Step 1
(d) If the relative molecular mass of compound X is 84000 units, determine the value of n (C = 12, H = 1)
(e) Write an equation that leads to the formation of substance E.
(f) State and explain the observation made when substances F and CH2=CH2 are burnt in excess air.
Date posted: September 3, 2019. Answers (1)
- A form one student crushed banana leaves with water and left the mixture for some days. He found that the mixture had fermented. He suspected...(Solved)
A form one student crushed banana leaves with water and left the mixture for some days. He found that the mixture had fermented. He suspected that the mixture had been contaminated with ethanol which has a boiling point of 780C while water has a boiling point of 1000C. The student then set up the apparatus below to separate the mixture.
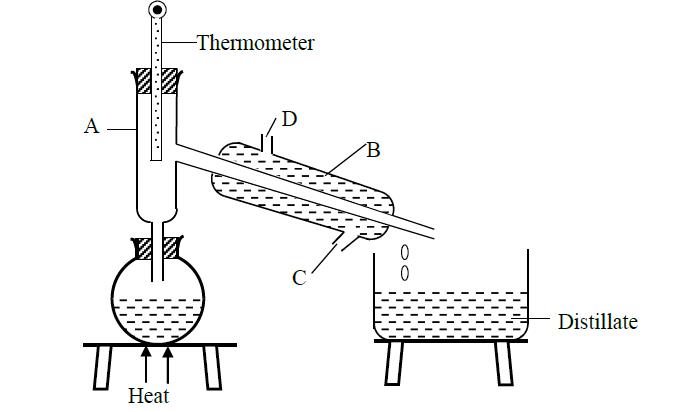
(i) Name the piece of apparatus labelled B.
(ii) What is the purpose of the thermometer in the set up?
(iii) At what point of apparatus B should the tap water be connected. Explain.
(iv) Name the part labelled A and state its function
(v) Which liquid was collected first? Explain
(vi) What is the name given to the above method of separating mixtures?
(vii) What property of the components of the mixture makes it possible for the components to be separated by the method?
(viii) State two applications of the above method of separation.
(b) A form two student was supplied with a liquid suspected to be water.
(i) Describe one chemical test that would be carried out to show that the liquid was water.
(ii) How would it have been proved that the liquid is pure water?
Date posted: September 3, 2019. Answers (1)
- The grid below is part of the periodic table. Letters are not actual symbols. Study it and answer the
questions that follow.(Solved)
The grid below is part of the periodic table. Letters are not actual symbols. Study it and answer the
questions that follow.
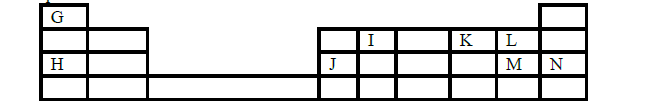
(i) Give the letters representing atoms that can form a singly-charged anion.
(ii) Identify the most electromagnetic element in the grid. Explain.
(iii) Identify the strongest reducing agent.
(iv) Write the formula of the most stable compound formed when J and K react
(v) Give the name of the type of bond in the compound formed in (iv) above.
(vi) Give the chemical family name of L and M.
(vii) Write the ionic equation for the reaction in which gas L is bubbled through a solution with ions of M.
(viii) Element P is alkaline earth metal and belongs to period 2. Indicate its position on the grid.
Date posted: September 3, 2019. Answers (1)
- Production of hydrogen iodide can be demonstrated by the equation below(Solved)
Production of hydrogen iodide can be demonstrated by the equation below
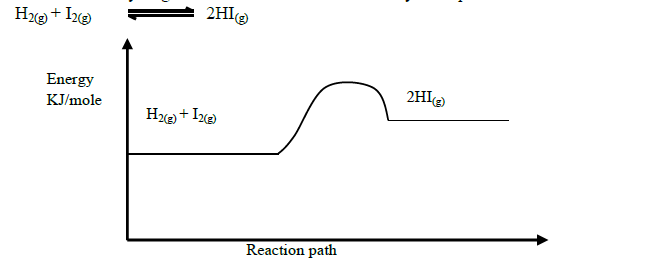
Explain how the following would affect the yield of hydrogen iodide.
(i) Increase in temperature
(ii) Decrease in pressure
Date posted: September 3, 2019. Answers (1)
- Study the following flow chart and answer the questions that follow.(Solved)
Study the following flow chart and answer the questions that follow.

(i) Name:
I. Alcohol R
II. Compound S
(ii) Name process T
Date posted: September 3, 2019. Answers (1)
- The table below shows elements in the halogen group of the periodic table. Study the table and answer the questions that follow.(Solved)
The table below shows elements in the halogen group of the periodic table. Study the table and answer the questions that follow.

(i) Name the element likely to be a solid at room temperature. Explain
(ii) Explain why the melting point increases from fluorine to iodine.
Date posted: September 3, 2019. Answers (1)
- Two gases A and B have relative densities of 1.98 and 2.90 respectively. They diffuse under the same conditions.
(i) Compare their rates of diffusion
(ii) Determine...(Solved)
Two gases A and B have relative densities of 1.98 and 2.90 respectively. They diffuse under the same conditions.
(i) Compare their rates of diffusion
(ii) Determine the relative molecular mass of A, given that the relative molecular mass of B is 64.
Date posted: September 3, 2019. Answers (1)
- During extraction of zinc metal, the ore is subjected to froth floatation. Give a reason why this process is necessary.(Solved)
During extraction of zinc metal, the ore is subjected to froth floatation. Give a reason why this process is necessary.
Date posted: September 3, 2019. Answers (1)
- When excess chlorine is bubbled through cold dilute sodium hydroxide solution, the resulting solution is a bleaching agent.
(a) Write a chemical equation for the reaction...(Solved)
When excess chlorine is bubbled through cold dilute sodium hydroxide solution, the resulting solution is a bleaching agent.
(a) Write a chemical equation for the reaction that produces the bleaching agent.
(b) Name the bleaching compound and show how it bleaches using an equation.
Date posted: September 3, 2019. Answers (1)
- In an experiment, excess magnesium ribbons were immersed in ethanoic acid and the gas evolved was measured at 10 seconds intervals.
(a) Write an equation for...(Solved)
In an experiment, excess magnesium ribbons were immersed in ethanoic acid and the gas evolved was measured at 10 seconds intervals.
(a) Write an equation for the reaction between Ethanoic acid and magnesium ribbon.
(b) Sketch a curve of volume of gas evolved against time for the above reaction.
(c) On the same axis above sketch the curve that would be obtained if hydrochloric acid was used. Label the curve 1.
Date posted: September 3, 2019. Answers (1)
- In order to prepare hydrogen gas in the laboratory a student set-up the apparatus shown in the diagram below. Study it and answer the questions...(Solved)
In order to prepare hydrogen gas in the laboratory a student set-up the apparatus shown in the diagram below. Study it and answer the questions that follow.
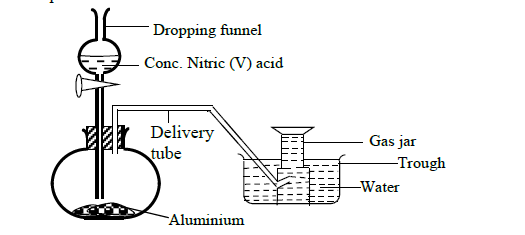
(a) Suggest why the student did not collect hydrogen gas.
(b) In a separate experiment the student reacted iron and hydrochloric acid to prepare hydrogen gas.
(i) Write an ionic equation for the reaction.
(ii) The hydrogen gas produced was found to have a foul smell. Suggest an explanation for this.
Date posted: September 3, 2019. Answers (1)
- Study the structure below.
C3H7COOC2H5
(a) Name the compound
(b) Name the compounds used to prepare the above compound.
(c) What is the identifying physical property of...(Solved)
Study the structure below.
C3H7COOC2H5
(a) Name the compound
(b) Name the compounds used to prepare the above compound.
(c) What is the identifying physical property of the above compound?
Date posted: September 3, 2019. Answers (1)
- Study the flow chart below and answer the questions that follow.(Solved)
Study the flow chart below and answer the questions that follow.
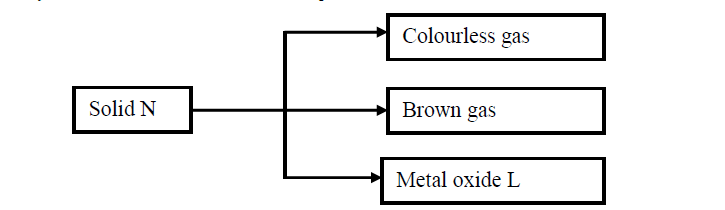
(a) Write the formula of the anion present in solid N.
(b) Solid N in the flow chart above burns in air with a red flame. Identify the
(i) Cation present in solid N
(ii) Metal oxide L
Date posted: September 3, 2019. Answers (1)
- Study the table below and answer the questions that follow.(Solved)
Study the table below and answer the questions that follow.
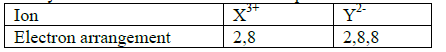
(a) Write the electronic arrangement of elements. X and Y
(b) Write the formula of the compound that would be formed between X and Y.
Date posted: September 3, 2019. Answers (1)
- Element R – 238 decays in series forming different nuclides as shown below.(Solved)
Element R – 238 decays in series forming different nuclides as shown below.
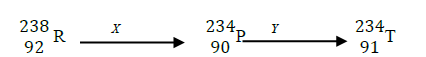
(i) Identify the type of decay X and Y
(ii) Give one use of radioactive isotopes in medicine
Date posted: September 3, 2019. Answers (1)
- Identify the particles which enable the following substances to conduct electricity.
(i) Aluminium metal
(ii) Molten lead (II) bromide(Solved)
Identify the particles which enable the following substances to conduct electricity.
(i) Aluminium metal
(ii) Molten lead (II) bromide
Date posted: September 3, 2019. Answers (1)
- The table below shows PH values of substances A, B, C and D. Study it and answer the questions that follow.(Solved)
The table below shows PH values of substances A, B, C and D. Study it and answer the questions that follow.

(a) Which substance is likely to be pure water
(b) Which solution contains the lowest concentration of hydrogen ions?
(c) In the equation below, identify the reagent that acts as a base. Give a reason for your answer.

Date posted: September 3, 2019. Answers (1)
- Give the I.U.P.A.C name of the oxide of nitrogen that:-
(i) Relights a glowing splint
(ii) Forms brown complex compound with acidified Iron (II) sulphate solution...(Solved)
Give the I.U.P.A.C name of the oxide of nitrogen that:-
(i) Relights a glowing splint
(ii) Forms brown complex compound with acidified Iron (II) sulphate solution
(iii) Reacts with water to form nitric (V) acid
Date posted: September 3, 2019. Answers (1)