- Use the standard electrode potentials for A, B, C, D and F given below to answer the questions that follow.(Solved)
Use the standard electrode potentials for A, B, C, D and F given below to answer the questions that follow.
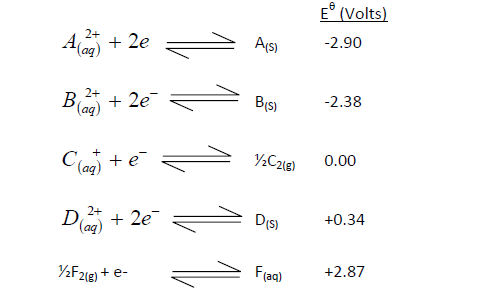
(i) Which element is likely to be hydrogen? Give a reason for your answer?
(ii) What is 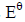 the strongest reducing agent?
the strongest reducing agent?
(iii) Calculate the e.m.f of the cell that would be formed when half cells of B and D are combined.
Date posted: September 5, 2019. Answers (1)
- Give two reasons why wood and charcoal are chosen for domestic heating.(Solved)
Give two reasons why wood and charcoal are chosen for domestic heating.
Date posted: September 5, 2019. Answers (1)
- Define heat value of a fuel.(Solved)
Define heat value of a fuel.
Date posted: September 5, 2019. Answers (1)
- The diagram below shows an energy level diagram for the formation of magnesium chloride. Study it and answer the questions that follow.(Solved)
The diagram below shows an energy level diagram for the formation of magnesium chloride. Study it and answer the questions that follow.
(i) State the enthalpy changes represented by A,B and C
(ii) What is the relationship between 
Date posted: September 5, 2019. Answers (1)
- A student from Nyeri High School wanted to determine the enthalpy change of combustion when a hydrocarbon with the formula C6H14 was burnt. The following...(Solved)
A student from Nyeri High School wanted to determine the enthalpy change of combustion when a hydrocarbon with the formula C6H14 was burnt. The following are the results of the experiment done.
Mass of water = 100g
Initial temperature = 18.0ºC
Final temperature = 58.0ºC
Mass of the hydrocarbon burned = 0.43g
Specific heat capacity of water = 4.2Jg-1k-1
(a) Write a balanced equation for the combustion of the hydrocarbon.
(b) (i) Calculate the amount of heat given out in kJ when 0.43g of the hydrocarbon burn in air.
(ii) Calculate the number of moles of the hydrocarbon that were burnt.
(iii) Calculate the molar enthalpy of combustion of the hydrocarbon.
(c) The theoretical value of the heat released when 1 mole of the hydrocarbon is burnt is 4194.7kJ/mol-1 Give two reasons why the value obtained from this experiment is less than the theoretical value.
Date posted: September 4, 2019. Answers (1)
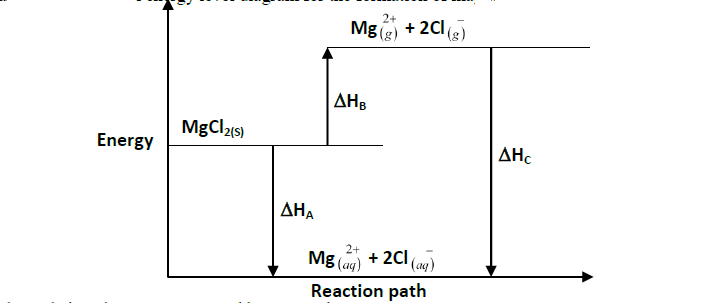
- The grid given below represents part of the periodic table. Study it and answer the questions that follow. Letters do not represent the actual symbols...(Solved)
The grid given below represents part of the periodic table. Study it and answer the questions that follow. Letters do not represent the actual symbols of the elements.
(i) What name is given to the group of elements to which C and F belong?
(ii) Which letter represents the element that is the least reactive?
(iii) What type of bond is formed when B and E react? Explain.
(iv) Write the formula of the compound formed when D and oxygen gas reacts.
(v) On the grid, indicate the position of element G which is in the third period of the periodic table and forms 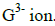
Date posted: September 4, 2019. Answers (1)
- Study the scheme below and answer the questions that follow.(Solved)
Study the scheme below and answer the questions that follow.
(i) Write an equation involving hot charcoal and dry carbon (IV) oxide gas.
(ii) Name gas X and state one chemical property of the gas.
Date posted: September 4, 2019. Answers (1)
- During extraction of copper, the ore is first concentrated and roasted to produce copper (I) sulphide.
(a) Write an equation for the reaction in which copper...(Solved)
During extraction of copper, the ore is first concentrated and roasted to produce copper (I) sulphide.
(a) Write an equation for the reaction in which copper (I) sulphide is produced by roasting the ore in air.
Date posted: September 4, 2019. Answers (1)
- Explain how a sample of CH3CH2CH2OH could be distinguished from a sample of CH3CH2COOH.(Solved)
Explain how a sample of CH3CH2CH2OH could be distinguished from a sample of CH3CH2COOH.
Date posted: September 4, 2019. Answers (1)
- Chlorine is used to prepare vinyl chloride (chloroethene) CH2 = CHCl.
(i) State why vinyl chloride (CH2 = CHCl) undergoes addition polymerization.
(ii) Name the polymer...(Solved)
Chlorine is used to prepare vinyl chloride (chloroethene) CH2 = CHCl.
(i) State why vinyl chloride (CH2 = CHCl) undergoes addition polymerization.
(ii) Name the polymer formed.
(iii) Complete the following equation to show how the two monomers combine during polymerization.
CH2 = CHCl + CH2 = CHCl----->
Date posted: September 4, 2019. Answers (1)
- State two reasons why tin coating is used in food cans.(Solved)
State two reasons why tin coating is used in food cans.
Date posted: September 4, 2019. Answers (1)
- The molecular formula mass of gas A is 28 and its empirical formula is CH2.
(a) Determine the molecular formula of gas A (C = 12.0,...(Solved)
The molecular formula mass of gas A is 28 and its empirical formula is CH2.
(a) Determine the molecular formula of gas A (C = 12.0, H = 1.0).
(b) Write the equation of the reaction between A and 1 mole of chlorine gas.
Date posted: September 4, 2019. Answers (1)
- When the oxide of metal Z is heated in the presence of metal X, it is reduced.The oxide of metal X is reduced by metal...(Solved)
When the oxide of metal Z is heated in the presence of metal X, it is reduced.
The oxide of metal X is reduced by metal Y. Arrange the three metals in order of increasing reactivity.
Date posted: September 4, 2019. Answers (1)
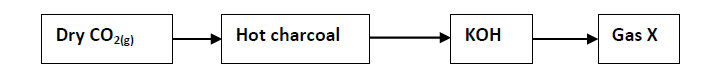
- Study the information in the table below and answer the questions that follow:(Solved)
Study the information in the table below and answer the questions that follow:
A mixture containing 40g salt G and 120g salt Y in 100g of water at 80ºC was cooled to 50ºC.
(a) Which salt crystallized out? Give reason.
(b) Calculate the mass of the salt that crystallized out.
Date posted: September 4, 2019. Answers (1)
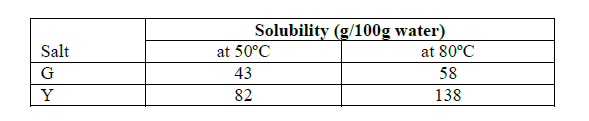
- Calculate the molar enthalpy of formation of ethyne (C2H2) given the following.(Solved)
Calculate the molar enthalpy of formation of ethyne (C2H2) given the following.
Date posted: September 4, 2019. Answers (1)
- Classify the following as either compounds or mixtures.(Solved)
Classify the following as either compounds or mixtures.

Date posted: September 4, 2019. Answers (1)
- Ethanol (CH3CH2OH) and dimethylether (CH3OCH3) are two compounds with the same molecular mass.
Explain why ethanol has a much higher boiling point (78.2ºC) than dimethylether (-24ºC).(Solved)
Ethanol (CH3CH2OH) and dimethylether (CH3OCH3) are two compounds with the same molecular mass.
Explain why ethanol has a much higher boiling point (78.2ºC) than dimethylether (-24ºC).
Date posted: September 4, 2019. Answers (1)
- Name the process that takes place when:
(i) Fats or oils are hydrolyzed using an alkali.
(ii) Sulphur is added to rubber in the manufacture of...(Solved)
Name the process that takes place when:
(i) Fats or oils are hydrolyzed using an alkali.
(ii) Sulphur is added to rubber in the manufacture of rubber tyres.
Date posted: September 4, 2019. Answers (1)
- In an experiment to investigate the conductivity of a substance a student used the set up shown below. The student noticed that the bulb did...(Solved)
In an experiment to investigate the conductivity of a substance a student used the set up shown below. The student noticed that the bulb did not light.
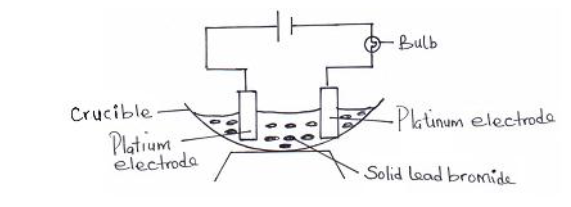
(i) What had been omitted in the set up?
(ii) Explain why the bulb lights up when the omission is corrected.
Date posted: September 4, 2019. Answers (1)
- Determine the oxidation number of:
(Solved)
Determine the oxidation number of:
(i) Manganese in KMnO4.
(ii) Chromium in 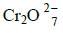
Date posted: September 4, 2019. Answers (1)