-
Illustrate bonding in carbon (II) oxide using dot and cross (C – 6, O – 8).
(Solved)
Illustrate bonding in carbon (II) oxide using dot and cross (C – 6, O – 8).
Date posted:
September 6, 2019
.
Answers (1)
-
The apparatus shown below were set-up to prepare and collect hydrogen sulphide gas.
(Solved)
The apparatus shown below were set-up to prepare and collect hydrogen sulphide gas.
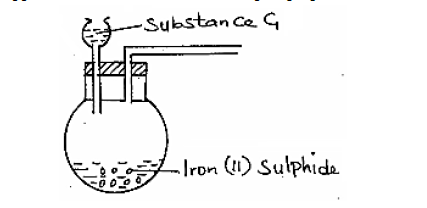
(a) Name substance G.
(b) Complete the set up to show how a dry sample of hydrogen sulphide gas is collected.
Date posted:
September 5, 2019
.
Answers (1)
-
An organic compound Y was analysed and found to contain carbon, hydrogen and oxygen only. 1.29g of Y on complete combustion gave 2.64g of carbon...
(Solved)
An organic compound Y was analysed and found to contain carbon, hydrogen and oxygen only. 1.29g of Y on complete combustion gave 2.64g of carbon (IV) oxide and 0.81g of water. Find the empirical formula of Y. (C = 12, H = 1, O = 16).
Date posted:
September 5, 2019
.
Answers (1)
-
The diagram below shows an experiment for investigating electrical conductivity in lead (II) iodide.
Study it and answer the questions that follow.
(Solved)
The diagram below shows an experiment for investigating electrical conductivity in lead (II) iodide.
Study it and answer the questions that follow.
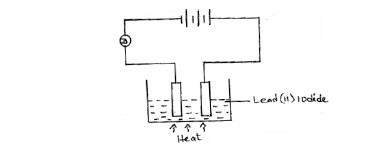
(a) On the diagram;
(i) Label the cathode.
(ii) Show the direction of movement of electrons.
(b) Write an equation for the reaction that takes place at the anode.
Date posted:
September 5, 2019
.
Answers (1)
-
Element X is found in period 3 group (IV) it consists of two isotopes 28X and QX. A sample of X was found to consist...
(Solved)
Element X is found in period 3 group (IV) it consists of two isotopes 28X and QX. A sample of X was found to consist of 90% of 28X if the relative atomic mass of X is 28.3, work out the number of neutrons in QX.
Date posted:
September 5, 2019
.
Answers (1)
-
Study the flow chart below and answer the questions that follow.
(Solved)
Study the flow chart below and answer the questions that follow.
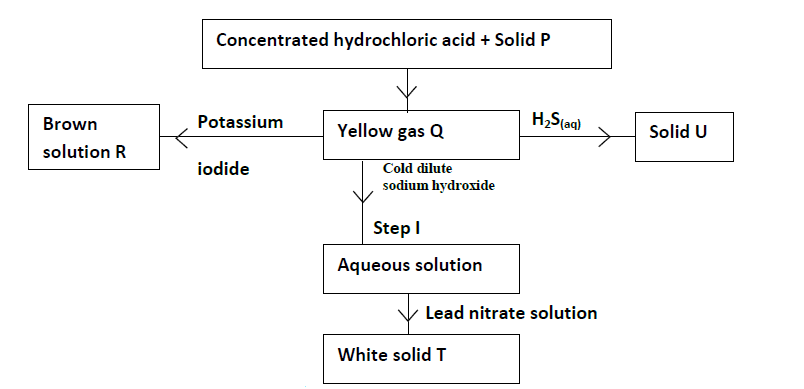
(a) Give the names of the following.
(i) Solid P
(ii) Solid U
(iii) Solid T
(b) Write the equation for the reaction taking place in Step I.
(c) Write the equation for the reaction between concentrated hydrochloric acid and solid P.
(d) Explain what would happen if hot concentrated sodium hydroxide was used in place of cold dilute sodium
hydroxide.
Date posted:
September 5, 2019
.
Answers (1)
-
The diagram below shows an energy level diagram for the formation of magnesium chloride. Study it and answer the questions that follow.
(Solved)
The diagram below shows an energy level diagram for the formation of magnesium chloride. Study it and answer the questions that follow.
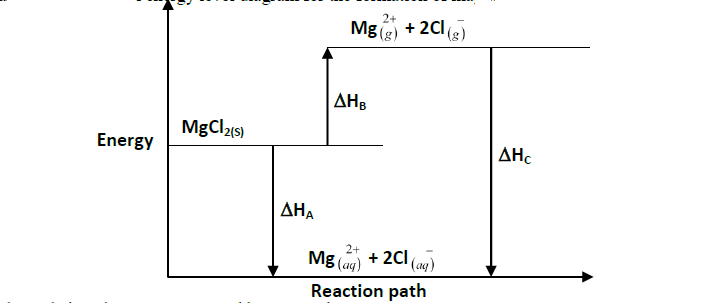
(i) State the enthalpy changes represented by A,B and C
(ii) What is the relationship between 
Date posted:
September 5, 2019
.
Answers (1)
-
Chlorine is used to prepare vinyl chloride (chloroethene) CH2 = CHCl.
(i) State why vinyl chloride (CH2 = CHCl) undergoes addition polymerization.
(ii) Name the polymer...
(Solved)
Chlorine is used to prepare vinyl chloride (chloroethene) CH2 = CHCl.
(i) State why vinyl chloride (CH2 = CHCl) undergoes addition polymerization.
(ii) Name the polymer formed.
(iii) Complete the following equation to show how the two monomers combine during polymerization.
CH2 = CHCl + CH2 = CHCl----->
Date posted:
September 4, 2019
.
Answers (1)
-
State two reasons why tin coating is used in food cans.
(Solved)
State two reasons why tin coating is used in food cans.
Date posted:
September 4, 2019
.
Answers (1)
-
When the oxide of metal Z is heated in the presence of metal X, it is reduced.The oxide of metal X is reduced by metal...
(Solved)
When the oxide of metal Z is heated in the presence of metal X, it is reduced.
The oxide of metal X is reduced by metal Y. Arrange the three metals in order of increasing reactivity.
Date posted:
September 4, 2019
.
Answers (1)
-
Study the information in the table below and answer the questions that follow:
(Solved)
Study the information in the table below and answer the questions that follow:
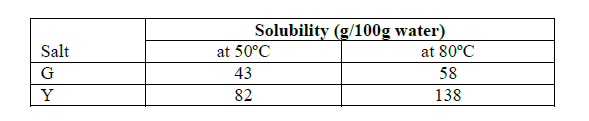
A mixture containing 40g salt G and 120g salt Y in 100g of water at 80ºC was cooled to 50ºC.
(a) Which salt crystallized out? Give reason.
(b) Calculate the mass of the salt that crystallized out.
Date posted:
September 4, 2019
.
Answers (1)
-
Calculate the molar enthalpy of formation of ethyne (C2H2) given the following.
(Solved)
Calculate the molar enthalpy of formation of ethyne (C2H2) given the following.
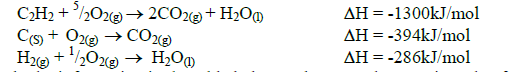
Date posted:
September 4, 2019
.
Answers (1)
-
In an experiment to investigate the conductivity of a substance a student used the set up shown below. The student noticed that the bulb did...
(Solved)
In an experiment to investigate the conductivity of a substance a student used the set up shown below. The student noticed that the bulb did not light.
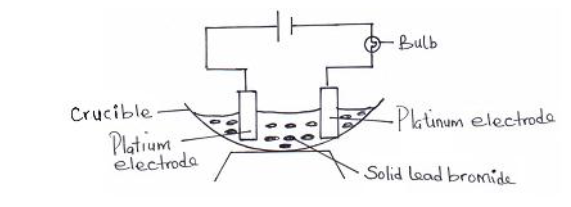
(i) What had been omitted in the set up?
(ii) Explain why the bulb lights up when the omission is corrected.
Date posted:
September 4, 2019
.
Answers (1)
-
Describe how a solid sample of lead (II) sulphate would be prepared using the following reagents: dilute nitric (V) acid, lead (II) carbonate solid sodium...
(Solved)
Describe how a solid sample of lead (II) sulphate would be prepared using the following reagents: dilute nitric (V) acid, lead (II) carbonate solid sodium sulphate and distilled water.
Date posted:
September 4, 2019
.
Answers (1)
-
The following results were obtained in an experiment to determine the heat of neutralization of 50cm3 2M hydrochloric acid and 50cm3 2M sodium hydroxide.
Mass of...
(Solved)
The following results were obtained in an experiment to determine the heat of neutralization of 50cm3 2M hydrochloric acid and 50cm3 2M sodium hydroxide.
Mass of plastic cup = 45.1g
Initial temperature of acid = 27.00C
Initial temperature of alkali = 23.00C
Mass of plastic cup + HCl + NaOH = 145.1g
Temperature of the mixture of acid and alkali = 38.50C
(a) Define heat of neutralization
(b) Write an ionic equation for the neutralization of hydrochloric acid and sodium hydroxide.
(i) The amount of heat produced during the experiment
(Specific heat capacity of solution = 4.2jg-1k-1, density of solution = 1gcm3)
(ii) Molar heat of neutralization for the reaction.
(c) Explain why the molar heat of neutralization of NaOH and Ethanoic acid of equal volume and molarity would be less than the value obtained in C (ii) above.
(d) Write down the thermochemical equation for reaction between NaOH and dilute hydrochloric acid
above
(e) Draw an energy level diagram for the neutralization reaction in (d) above.
Date posted:
September 4, 2019
.
Answers (1)
-
The chart shows the extraction of lead from its ore.
(Solved)
The chart shows the extraction of lead from its ore.
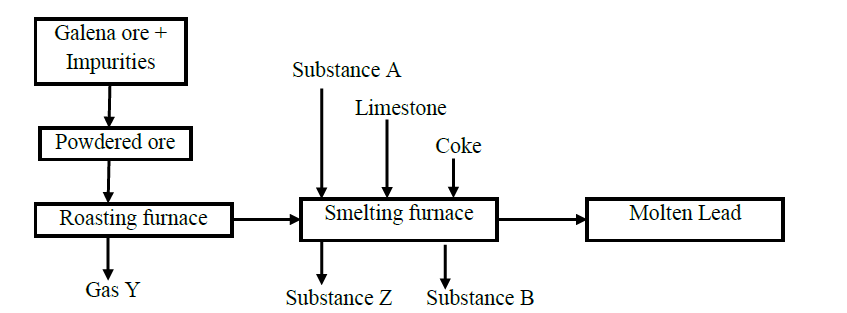
(a) Write the chemical formula of the chief ore.
(b) Name the possible impurities present in the ore.
(c) Why is it necessary for the ore to be converted into powder form?
(d) Identify process X and state it‟s significance
(e) Write equations for the reaction taking place in the
I. Roasting furnace
II. Formation of substance B
It is not advisable to use lead pipes in transporting drinking water.
Explain why.
(f) Identify one of the impurities present in molten lead obtained by the process.
(g) State one use of lead
Date posted:
September 4, 2019
.
Answers (1)
-
The flow chart shows some chemical reactions.
(Solved)
The flow chart shows some chemical reactions.

(a) Draw the structural formula and names of the following compounds.
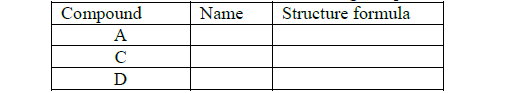
(b) Write the name of the processes that leads to the formation of substances A and F.

(c) Name the type of reaction and conditions required for the formation Step 1
(d) If the relative molecular mass of compound X is 84000 units, determine the value of n (C = 12, H = 1)
(e) Write an equation that leads to the formation of substance E.
(f) State and explain the observation made when substances F and CH2=CH2 are burnt in excess air.
Date posted:
September 3, 2019
.
Answers (1)
-
A form one student crushed banana leaves with water and left the mixture for some days. He found that the mixture had fermented. He suspected...
(Solved)
A form one student crushed banana leaves with water and left the mixture for some days. He found that the mixture had fermented. He suspected that the mixture had been contaminated with ethanol which has a boiling point of 780C while water has a boiling point of 1000C. The student then set up the apparatus below to separate the mixture.
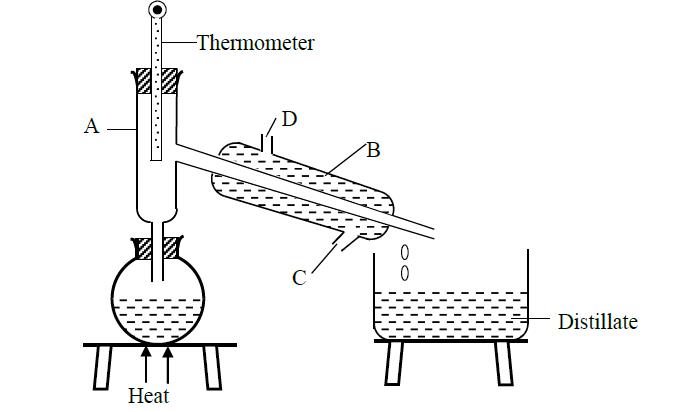
(i) Name the piece of apparatus labelled B.
(ii) What is the purpose of the thermometer in the set up?
(iii) At what point of apparatus B should the tap water be connected. Explain.
(iv) Name the part labelled A and state its function
(v) Which liquid was collected first? Explain
(vi) What is the name given to the above method of separating mixtures?
(vii) What property of the components of the mixture makes it possible for the components to be separated by the method?
(viii) State two applications of the above method of separation.
(b) A form two student was supplied with a liquid suspected to be water.
(i) Describe one chemical test that would be carried out to show that the liquid was water.
(ii) How would it have been proved that the liquid is pure water?
Date posted:
September 3, 2019
.
Answers (1)
-
Production of hydrogen iodide can be demonstrated by the equation below
(Solved)
Production of hydrogen iodide can be demonstrated by the equation below
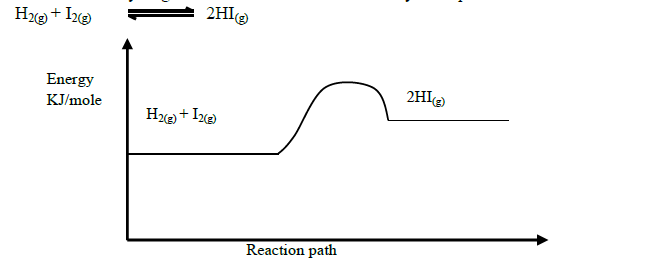
Explain how the following would affect the yield of hydrogen iodide.
(i) Increase in temperature
(ii) Decrease in pressure
Date posted:
September 3, 2019
.
Answers (1)
-
The table below shows elements in the halogen group of the periodic table. Study the table and answer the questions that follow.
(Solved)
The table below shows elements in the halogen group of the periodic table. Study the table and answer the questions that follow.

(i) Name the element likely to be a solid at room temperature. Explain
(ii) Explain why the melting point increases from fluorine to iodine.
Date posted:
September 3, 2019
.
Answers (1)