- The solubilities of two salts D and E are given in the following table in each case the solubility is expressed as grammes per 100g...(Solved)
The solubilities of two salts D and E are given in the following table in each case the solubility is expressed as grammes per 100g of water.

(a) Using these data plot solubility curves for D and E on the same grid.
(b) Use your graph to answer the following questions:
(i) At what temperature are the solubilities of the two salts equal?
(ii) Estimate the solubility of salt D at OºC.
(iii) A saturated solution of E in 50g of water at 25ºC was evaporated to dryness. What was the mass of the residue?
(iv) Two separate 100g of water are saturated at 75ºC, one with D and the other with E. What is the difference in mass between
the two solutions?
(v) The saturated solution obtained were each cooled to 20ºC.
I Calculate the total mass of the two salts precipitated.
II Calculate the mass of each salt dissolved at saturation in 20g of water at 20ºC.
Date posted: September 6, 2019. Answers (1)
- 1g of element T was completely converted to its chloride, TCl2 The mass of the chloride formed was 3.96g. Calculate the relative atomic mass of...(Solved)
1g of element T was completely converted to its chloride, TCl2 The mass of the chloride formed was 3.96g. Calculate the relative atomic mass of element T. (Cl = 35.5).
Date posted: September 6, 2019. Answers (1)
- In an experiment to prepare hydrogen gas using magnesium ribbon and dilute hydrochloric acid, a student plotted volume of hydrogen gas against time as shown...(Solved)
In an experiment to prepare hydrogen gas using magnesium ribbon and dilute hydrochloric acid, a student plotted volume of hydrogen gas against time as shown in the sketch below.
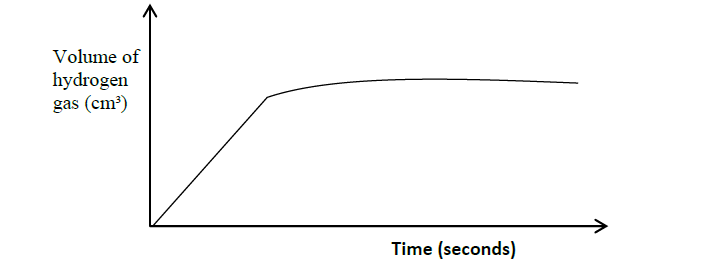
(i) On the same axes, sketch the curve that would be obtained if a few crystals of copper (II) sulphate are added and label it curve C.
(ii) What would be the function of copper (II) sulphate in the reaction?
Date posted: September 6, 2019. Answers (1)
- Below is part of a synthetic polymer. Study it and answer the questions that follow.(Solved)
Below is part of a synthetic polymer. Study it and answer the questions that follow.
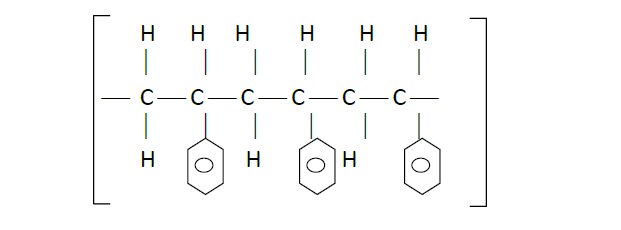
(i) Draw the structure of its monomer.
(ii) Determine the number of monomers making the above compound if its relative molecular mass is 104,000. The benzene ring has six carbon atoms and five hydrogen atoms (C = 12, H = 1).
Date posted: September 6, 2019. Answers (1)
- Illustrate bonding in carbon (II) oxide using dot and cross (C – 6, O – 8).(Solved)
Illustrate bonding in carbon (II) oxide using dot and cross (C – 6, O – 8).
Date posted: September 6, 2019. Answers (1)
- Radon Ra undergoes alpha decay to form lead, taking 15 days for the original mass to reduce to 6.25%.(Solved)
 Ra undergoes alpha decay to form lead, taking 15 days for the original mass to reduce to 6.25%.
Ra undergoes alpha decay to form lead, taking 15 days for the original mass to reduce to 6.25%.
(a) Write the nuclear equation for the reaction.
(b) Calculate the half-life of radon.
Date posted: September 5, 2019. Answers (1)
- The apparatus shown below were set-up to prepare and collect hydrogen sulphide gas.(Solved)
The apparatus shown below were set-up to prepare and collect hydrogen sulphide gas.
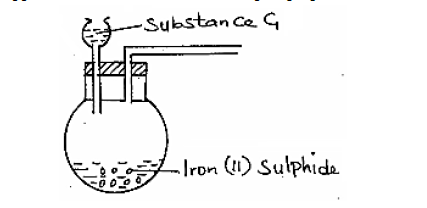
(a) Name substance G.
(b) Complete the set up to show how a dry sample of hydrogen sulphide gas is collected.
Date posted: September 5, 2019. Answers (1)
- An equilibrium exists between the reactants and products as shown in the equation below.(Solved)
An equilibrium exists between the reactants and products as shown in the equation below.
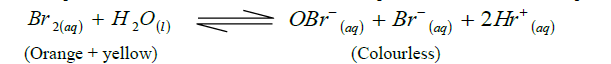
(i) Select the species that acts as an acid. Explain.
(ii) State and explain the observations made when aqueous sodium hydroxide solution is added to the above
equilibrium.
Date posted: September 5, 2019. Answers (1)
- An organic compound Y was analysed and found to contain carbon, hydrogen and oxygen only. 1.29g of Y on complete combustion gave 2.64g of carbon...(Solved)
An organic compound Y was analysed and found to contain carbon, hydrogen and oxygen only. 1.29g of Y on complete combustion gave 2.64g of carbon (IV) oxide and 0.81g of water. Find the empirical formula of Y. (C = 12, H = 1, O = 16).
Date posted: September 5, 2019. Answers (1)
- The scheme below represents the manufacture of a cleansing agent M.(Solved)
The scheme below represents the manufacture of a cleansing agent M.
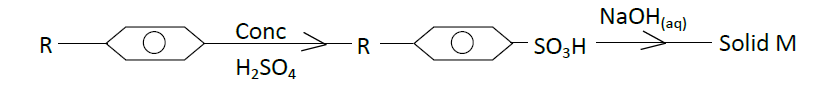
(a) (i) Draw the structure of M.
(ii) To which type of cleansing agent does M belong?
Date posted: September 5, 2019. Answers (1)
- Study the flow chart below and answer the questions that follow.(Solved)
Study the flow chart below and answer the questions that follow.
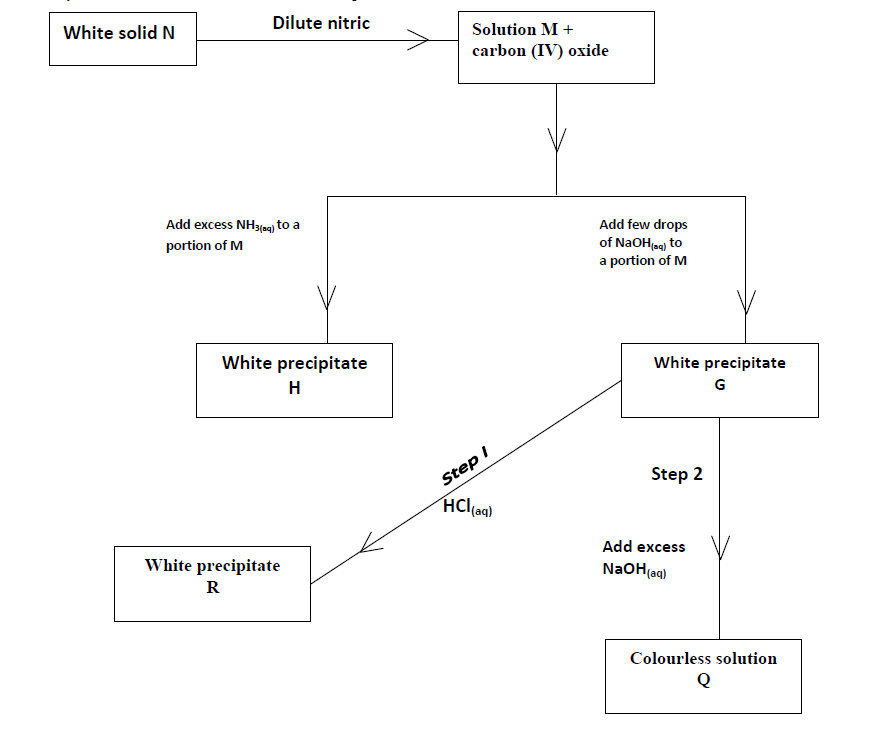
(a) Identify solid N.
(b) Write down the equation for the reaction that leads to the formation of solution Q from the white precipitate G.
(c) State the property of precipitate G that is demonstrated by Step 1 and 2.
Date posted: September 5, 2019. Answers (1)
- The diagram below shows an experiment for investigating electrical conductivity in lead (II) iodide.
Study it and answer the questions that follow.(Solved)
The diagram below shows an experiment for investigating electrical conductivity in lead (II) iodide.
Study it and answer the questions that follow.
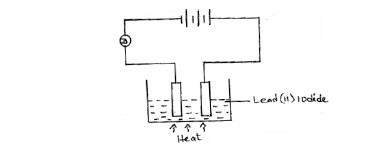
(a) On the diagram;
(i) Label the cathode.
(ii) Show the direction of movement of electrons.
(b) Write an equation for the reaction that takes place at the anode.
Date posted: September 5, 2019. Answers (1)
- Given the following bond energies.(Solved)
Given the following bond energies.

Calculate the enthalpy change of hydrogenation of ethene.
Date posted: September 5, 2019. Answers (1)
- Study the table below and answer the questions that follow:(Solved)
Study the table below and answer the questions that follow:
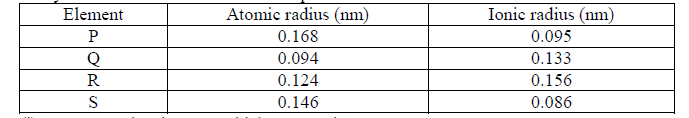
(i) State the elements which are metals.
(ii) Identify the strongest reducing agent. Give a reason.
Date posted: September 5, 2019. Answers (1)
- Element X is found in period 3 group (IV) it consists of two isotopes 28X and QX. A sample of X was found to consist...(Solved)
Element X is found in period 3 group (IV) it consists of two isotopes 28X and QX. A sample of X was found to consist of 90% of 28X if the relative atomic mass of X is 28.3, work out the number of neutrons in QX.
Date posted: September 5, 2019. Answers (1)
- The table below shows atomic numbers of four elements W, X, Y and Z.(Solved)
The table below shows atomic numbers of four elements W, X, Y and Z.

(a) Write electron arrangement of the ion of Z.
(b) (i) Write the formula of the compound formed between W and X.
(ii) Name the bond(s) and structure of the compound in (i) above.
Date posted: September 5, 2019. Answers (1)
- Study the flow chart below and answer the questions that follow.(Solved)
Study the flow chart below and answer the questions that follow.
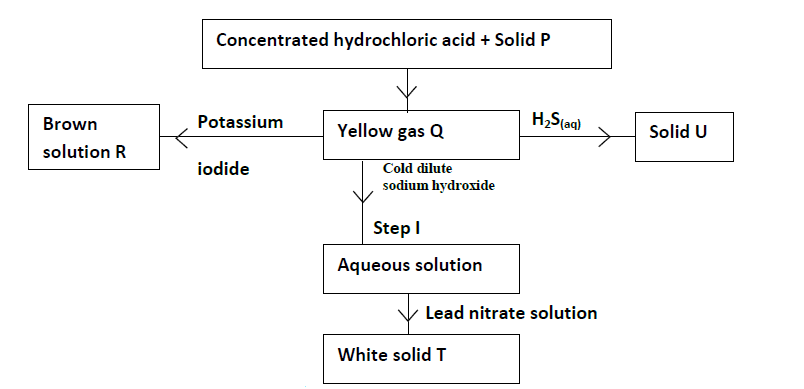
(a) Give the names of the following.
(i) Solid P
(ii) Solid U
(iii) Solid T
(b) Write the equation for the reaction taking place in Step I.
(c) Write the equation for the reaction between concentrated hydrochloric acid and solid P.
(d) Explain what would happen if hot concentrated sodium hydroxide was used in place of cold dilute sodium
hydroxide.
Date posted: September 5, 2019. Answers (1)
- Draw the structure of the following compound 2-methylbutan-l-ol.(Solved)
Draw the structure of the following compound 2-methylbutan-l-ol.
Date posted: September 5, 2019. Answers (1)
- Name the following organic compound.(Solved)
Name the following organic compound.
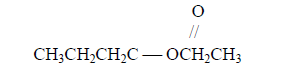
Date posted: September 5, 2019. Answers (1)
- During electrolysis of aqueous copper (II) sulphate using copper electrodes, a current of 0.2 amperes was passed through the cell for 5 hours.
Determine the change...(Solved)
During electrolysis of aqueous copper (II) sulphate using copper electrodes, a current of 0.2 amperes was passed through the cell for 5 hours.
Determine the change in mass of the cathode that occurred as a result of the electrolysis process
(Cu = 64, IF = 96,500C).
Date posted: September 5, 2019. Answers (1)