- Coke – to reduce PbO to Pb
- Limestone – to remove silica as slag.
- Scrap iron – to reduce unleaded PbS to Pb.
maurice.mutuku answered the question on September 6, 2019 at 09:09
- (a) State why it's necessary to dry the hydrogen gas before igniting it.
(i) State the precaution that must be taken before igniting the hydrogen.(Solved)
(a) State why it's necessary to dry the hydrogen gas before igniting it.
(i) State the precaution that must be taken before igniting the hydrogen.
Date posted: September 6, 2019. Answers (1)
- Hydrogen sulphide gas was bubbled through a solution of zinc nitrate for sometime.
(i) State the observations made.
(ii) Where should the experiment be carried out...(Solved)
Hydrogen sulphide gas was bubbled through a solution of zinc nitrate for sometime.
(i) State the observations made.
(ii) Where should the experiment be carried out and why?
Date posted: September 6, 2019. Answers (1)
- The volume of a sample of nitrogen gas at a temperature of 298k and 600 minHg pressures was 4.8 x 10-2m3
Calculate the temperature at which...(Solved)
The volume of a sample of nitrogen gas at a temperature of 298k and 600 minHg pressures was 4.8 x 10-2m3
Calculate the temperature at which the volume of the gas would be 3.2x102m3 if pressure is constant.
Date posted: September 6, 2019. Answers (1)
- Ammonia gas is used to manufacture nitric (V) acid as shown below.(Solved)
Ammonia gas is used to manufacture nitric (V) acid as shown below.
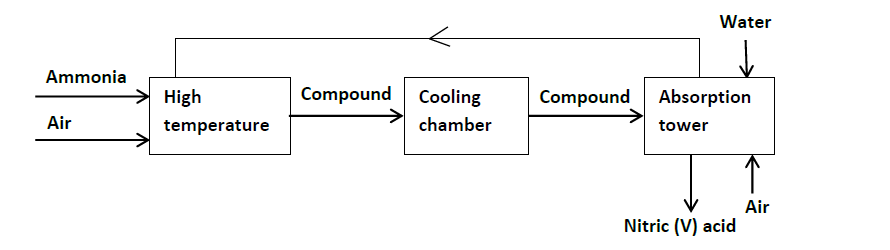
(i) Name the catalyst used in the above process.
(ii) Identify compound U.
(iii) Write the equation for the reaction that took place in the absorption tower.
(iv) Ammonia and nitric (V) acid are used in the manufacture of ammonium nitrate fertilizer, calculate the amount of the fertilizer manufactured per day, if the daily consumption of ammonia is 2400kg. Assume that the factory is 100%
efficient. (N = 14, H = 1, O = 16).
Date posted: September 6, 2019. Answers (1)
- The diagram below shows an incomplete set-up used to prepare and collect ammonia gas.(Solved)
The diagram below shows an incomplete set-up used to prepare and collect ammonia gas.
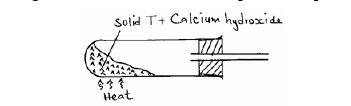
(i) Name solid T.
(ii) Write an equation for the reaction that occurred when a mixture of solid T and calcium hydroxide was heated.
(iii) Complete the diagram to show how a dry sample of ammonia gas can be collected.
Date posted: September 6, 2019. Answers (1)
- In an experiment to determine the molar heat of reaction when zinc displaces copper, 0.4g of zinc powder were added to 25.0cm³ of 2.0M copper...(Solved)
In an experiment to determine the molar heat of reaction when zinc displaces copper, 0.4g of zinc powder were added to 25.0cm³ of 2.0M copper (II) sulphate solution. The temperature of copper (II) sulphate solution was 24ºC, while that of the mixture was 36ºC.
(i) Other than increase in temperature, state and explain the observations which were made during the reaction.
(ii) Calculate the heat change during the reaction. (Specific heat capacity of the solution = 4.2Jgˉ¹Kˉ¹ and the density of the solution = 1g/cm³.
(iii) Determine the molar heat of displacement of copper by zinc. (Zn = 65).
Date posted: September 6, 2019. Answers (1)
- Methanol is manufactured from carbon (IV) oxide and hydrogen gas according to the equation.(Solved)
Methanol is manufactured from carbon (IV) oxide and hydrogen gas according to the equation.
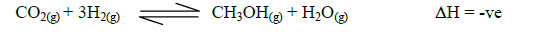
The reaction is carried out in the presence of a chromium catalyst at 400ºC and 30Kpa under these conditions, an equilibrium is reached when 2% of the carbon (IV) oxide is converted to methanol.
(i) Explain how the yield of methanol would be affected if; the manufacturing process above is carried out at, 200ºC and a pressure of 30Kpa.
(ii) Using a more efficient catalyst.
Date posted: September 6, 2019. Answers (1)
- Study the flow diagram below and answer the questions that follow.(Solved)
Study the flow diagram below and answer the questions that follow.
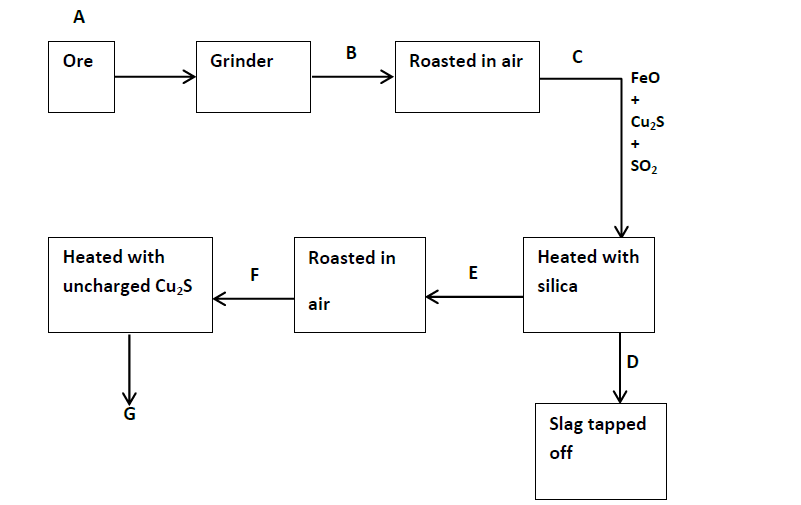
(a) Give the names of the two ores that can be used in the above process in Stage A.
(b) What process takes place in Stage B?
(c) Give the equation for the formation of the slag that is tapped of in Stage D.
What is the name of the slag?
(d) What are the names of the products formed in Stage G?
(e) What are the main impurities that are contained in the copper obtained in Stage G.
(f) Draw a well labelled diagram of the set-up of apparatus that would be used to purify the copper obtained in Stage G.
Date posted: September 6, 2019. Answers (1)
- The standard reduction potentials for five half cells are shown in the table below.
Study it and answer the questions that follow. The letters do not...(Solved)
The standard reduction potentials for five half cells are shown in the table below.
Study it and answer the questions that follow. The letters do not represent the actual symbols of the elements.
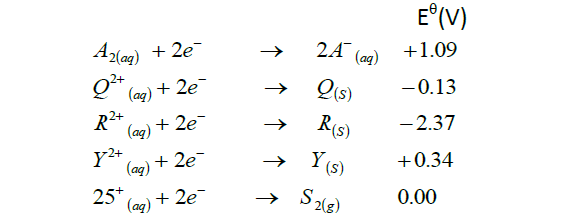
(i) With a reason identify the strongest reducing agent.
(ii) Which element is likely to be hydrogen. Explain.
(iii) Write an equation for the cell formed when Q and Y half cells are joined.
(iv) Calculate the e.m.f of the cell in (iii) above.
Date posted: September 6, 2019. Answers (1)
- The solubilities of two salts D and E are given in the following table in each case the solubility is expressed as grammes per 100g...(Solved)
The solubilities of two salts D and E are given in the following table in each case the solubility is expressed as grammes per 100g of water.

(a) Using these data plot solubility curves for D and E on the same grid.
(b) Use your graph to answer the following questions:
(i) At what temperature are the solubilities of the two salts equal?
(ii) Estimate the solubility of salt D at OºC.
(iii) A saturated solution of E in 50g of water at 25ºC was evaporated to dryness. What was the mass of the residue?
(iv) Two separate 100g of water are saturated at 75ºC, one with D and the other with E. What is the difference in mass between
the two solutions?
(v) The saturated solution obtained were each cooled to 20ºC.
I Calculate the total mass of the two salts precipitated.
II Calculate the mass of each salt dissolved at saturation in 20g of water at 20ºC.
Date posted: September 6, 2019. Answers (1)
- 1g of element T was completely converted to its chloride, TCl2 The mass of the chloride formed was 3.96g. Calculate the relative atomic mass of...(Solved)
1g of element T was completely converted to its chloride, TCl2 The mass of the chloride formed was 3.96g. Calculate the relative atomic mass of element T. (Cl = 35.5).
Date posted: September 6, 2019. Answers (1)
- In an experiment to prepare hydrogen gas using magnesium ribbon and dilute hydrochloric acid, a student plotted volume of hydrogen gas against time as shown...(Solved)
In an experiment to prepare hydrogen gas using magnesium ribbon and dilute hydrochloric acid, a student plotted volume of hydrogen gas against time as shown in the sketch below.
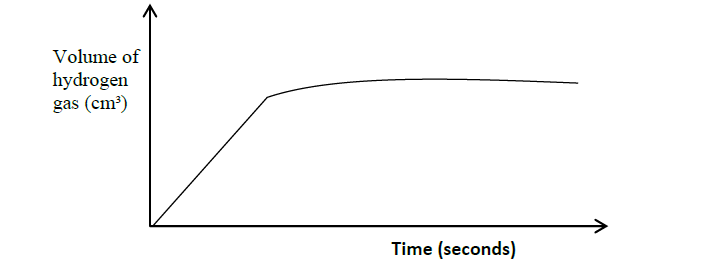
(i) On the same axes, sketch the curve that would be obtained if a few crystals of copper (II) sulphate are added and label it curve C.
(ii) What would be the function of copper (II) sulphate in the reaction?
Date posted: September 6, 2019. Answers (1)
- Below is part of a synthetic polymer. Study it and answer the questions that follow.(Solved)
Below is part of a synthetic polymer. Study it and answer the questions that follow.
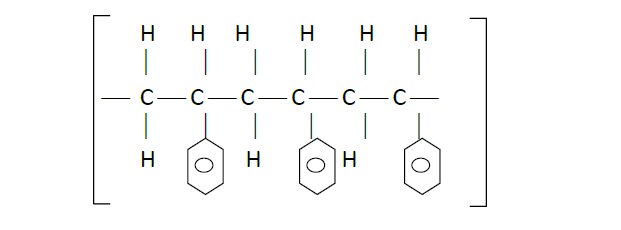
(i) Draw the structure of its monomer.
(ii) Determine the number of monomers making the above compound if its relative molecular mass is 104,000. The benzene ring has six carbon atoms and five hydrogen atoms (C = 12, H = 1).
Date posted: September 6, 2019. Answers (1)
- Illustrate bonding in carbon (II) oxide using dot and cross (C – 6, O – 8).(Solved)
Illustrate bonding in carbon (II) oxide using dot and cross (C – 6, O – 8).
Date posted: September 6, 2019. Answers (1)
- Radon Ra undergoes alpha decay to form lead, taking 15 days for the original mass to reduce to 6.25%.(Solved)
 Ra undergoes alpha decay to form lead, taking 15 days for the original mass to reduce to 6.25%.
Ra undergoes alpha decay to form lead, taking 15 days for the original mass to reduce to 6.25%.
(a) Write the nuclear equation for the reaction.
(b) Calculate the half-life of radon.
Date posted: September 5, 2019. Answers (1)
- The apparatus shown below were set-up to prepare and collect hydrogen sulphide gas.(Solved)
The apparatus shown below were set-up to prepare and collect hydrogen sulphide gas.
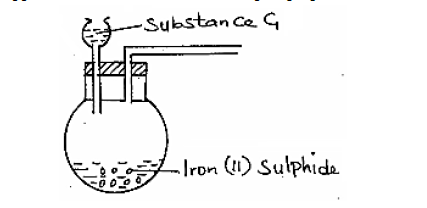
(a) Name substance G.
(b) Complete the set up to show how a dry sample of hydrogen sulphide gas is collected.
Date posted: September 5, 2019. Answers (1)
- An equilibrium exists between the reactants and products as shown in the equation below.(Solved)
An equilibrium exists between the reactants and products as shown in the equation below.
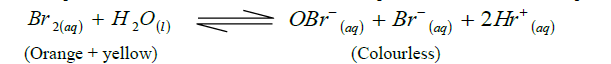
(i) Select the species that acts as an acid. Explain.
(ii) State and explain the observations made when aqueous sodium hydroxide solution is added to the above
equilibrium.
Date posted: September 5, 2019. Answers (1)
- An organic compound Y was analysed and found to contain carbon, hydrogen and oxygen only. 1.29g of Y on complete combustion gave 2.64g of carbon...(Solved)
An organic compound Y was analysed and found to contain carbon, hydrogen and oxygen only. 1.29g of Y on complete combustion gave 2.64g of carbon (IV) oxide and 0.81g of water. Find the empirical formula of Y. (C = 12, H = 1, O = 16).
Date posted: September 5, 2019. Answers (1)
- The scheme below represents the manufacture of a cleansing agent M.(Solved)
The scheme below represents the manufacture of a cleansing agent M.
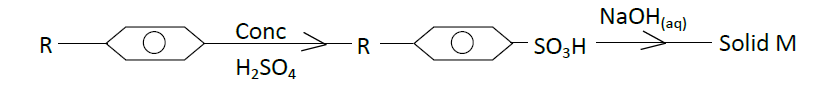
(a) (i) Draw the structure of M.
(ii) To which type of cleansing agent does M belong?
Date posted: September 5, 2019. Answers (1)
- Study the flow chart below and answer the questions that follow.(Solved)
Study the flow chart below and answer the questions that follow.
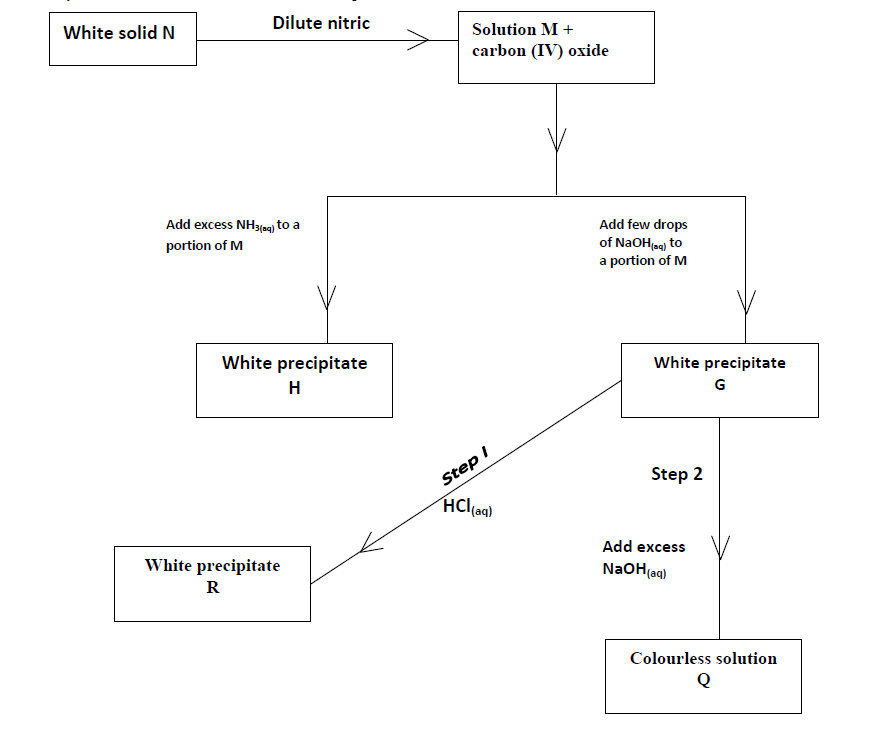
(a) Identify solid N.
(b) Write down the equation for the reaction that leads to the formation of solution Q from the white precipitate G.
(c) State the property of precipitate G that is demonstrated by Step 1 and 2.
Date posted: September 5, 2019. Answers (1)