-
The diagram below shows a graph of force against extension for a certain spring.
(Solved)
The diagram below shows a graph of force against extension for a certain spring.
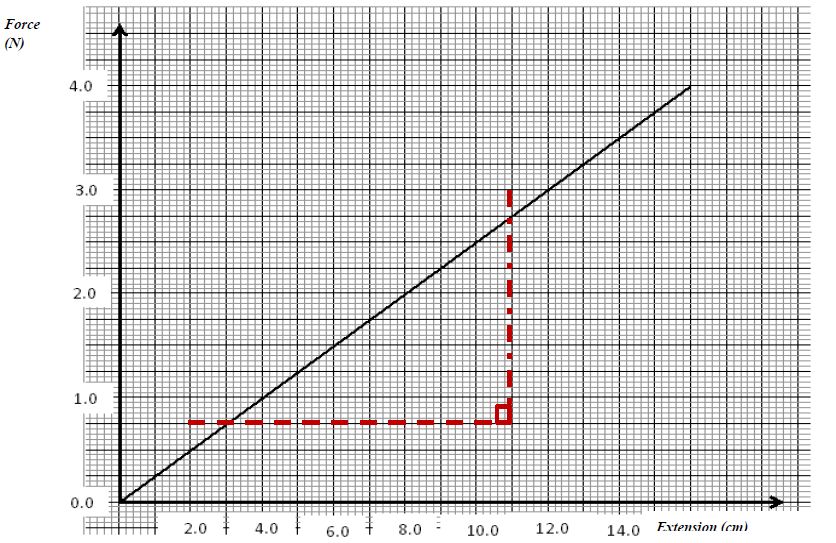
(i) What is the spring constant of the spring?
(ii) What force would cause two such springs placed side by side to stretch by 10cm
Date posted:
September 6, 2019
.
Answers (1)
-
The height of a mountain is 1360m. The barometer reading at the base of the mountain is 74cmHg. Given that the densities of mercury and...
(Solved)
The height of a mountain is 1360m. The barometer reading at the base of the mountain is 74cmHg. Given that the densities of mercury and air are 13,600Kgm-3 and 1.25Kgm-3 respectively, determine the barometer reading at the top of the mountain.
Date posted:
September 6, 2019
.
Answers (1)
-
The figure below is a simple hydraulic machine used to raise heavy loads.
(Solved)
The figure below is a simple hydraulic machine used to raise heavy loads.
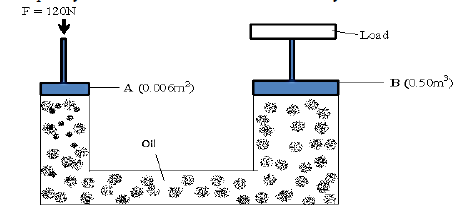
Calculate;
(i) The pressure exerted on the oil by the force applied at A
(ii) The load raised at B
(iii) Give two properties which make the oil suitable for use in this machine
Date posted:
September 6, 2019
.
Answers (1)
-
A stone of mass 40g is tied to the end of a string 50cm long such that it is 20m above the ground at its...
(Solved)
A stone of mass 40g is tied to the end of a string 50cm long such that it is 20m above the ground at its lowest level as shown in the diagram below. It is whirled in a vertical circle at 2rev/s.
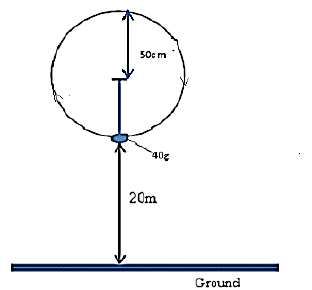
(i) If the string breaks at its lowest level as shown, what is the velocity with which it travels?
Calculate the maximum tension in the string.
(ii) Calculate the maximum tension in the string.
(iii) Determine the maximum horizontal distance it travels from the breaking point
Date posted:
September 6, 2019
.
Answers (1)
-
Using Kinetic theory of matter, explain why solids expand when heated.
(Solved)
Using Kinetic theory of matter, explain why solids expand when heated.
Date posted:
September 6, 2019
.
Answers (1)
-
The diagram below shows a braking system.Why is the master piston, made smaller than the slave piston?
(Solved)
The diagram below shows a braking system.Why is the master piston, made smaller than the slave piston?

Date posted:
September 6, 2019
.
Answers (1)
-
The diagram below shows a micrometer screw gauge. What is the reading in SI units?
(Solved)
The diagram below shows a micrometer screw gauge. What is the reading in SI units?
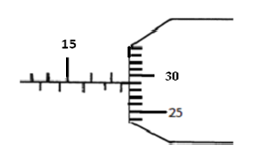
Date posted:
September 6, 2019
.
Answers (1)
-
The figure below shows photocell used in a set-up for a burglar alarm.
(Solved)
The figure below shows photocell used in a set-up for a burglar alarm.
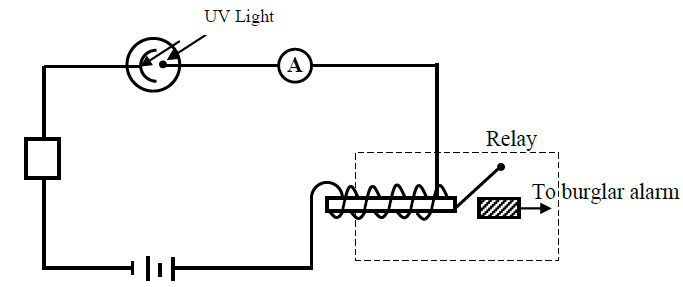
(i) Give a reason why the photocell is usually evacuated.
(ii) State the function of the resistor R in this circuit
(iii) Explain why a particular radiation such as ultra-violet light is used to strike a given cathode surface.
(iv) Explain how the set-up in the figure can be used as a burglar alarm.
Date posted:
September 6, 2019
.
Answers (1)
-
The graph below shows the variation of 1/v and 1/u in an experiment to determine the focal length of a lens.
(Solved)
The graph below shows the variation of 1/v and 1/u in an experiment to determine the focal length of a lens.
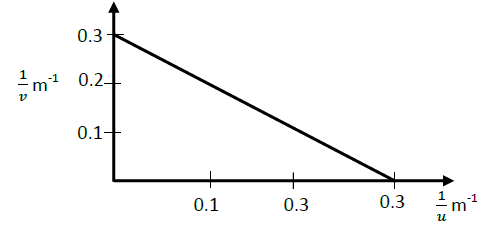
(i) Use the graph to determine the focal length
(ii) What is the power of the lens used?
Date posted:
September 6, 2019
.
Answers (1)
-
A form two student from Kimomo Secondary School found his dry cells leaking on removing from his torch. What would be the possible cause of...
(Solved)
A form two student from Kimomo Secondary School found his dry cells leaking on removing from his torch. What would be the possible cause of the leakage?
Date posted:
September 6, 2019
.
Answers (1)
-
A vibrator is sending out 8 ripples per second across a ripple water tank. The ripples are observed to be 4cm apart. Calculate the velocity...
(Solved)
A vibrator is sending out 8 ripples per second across a ripple water tank. The ripples are observed to be 4cm apart. Calculate the velocity of the ripples
Date posted:
September 6, 2019
.
Answers (1)
-
The figure below shows a positively charged metal plate with an earthing connection. State and explain the final charge of the plate.
(Solved)
The figure below shows a positively charged metal plate with an earthing connection. State and explain the final charge of the plate.
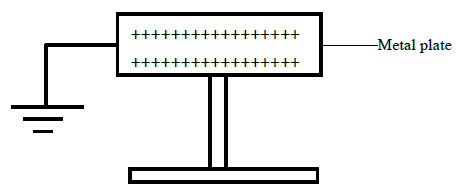
Date posted:
September 6, 2019
.
Answers (1)
-
The figure below shows a buoy of capacity 40 litres and mass 10kg. It is held in position in sea water of
density 1.04g/cm3 by a...
(Solved)
The figure below shows a buoy of capacity 40 litres and mass 10kg. It is held in position in sea water of
density 1.04g/cm3 by a light cable fixed to the bottom so that ¾ of its volume is below the water surface.
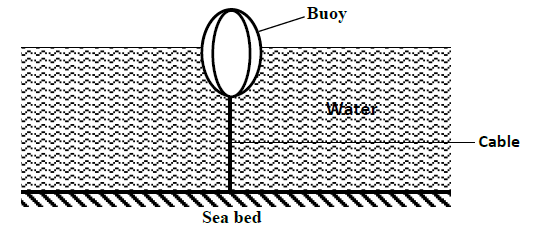
Determine the tension in the cable.
Date posted:
September 6, 2019
.
Answers (1)
-
A car decelerates uniformly from a velocity of 20m/s to rest in 4 seconds. It takes 4 seconds to reverse with uniform acceleration to its...
(Solved)
A car decelerates uniformly from a velocity of 20m/s to rest in 4 seconds. It takes 4 seconds to reverse with uniform acceleration to its original starting point.
(i) Sketch a velocity time graph for the motion of the car.
(ii) Use your sketch in c (i) to determine the total displacement of the car.
Date posted:
September 6, 2019
.
Answers (1)
-
The figure below shows the displacement – time graph of the motion of a particle.
State the nature of the motion of the particle between?
(i)...
(Solved)
The figure below shows the displacement – time graph of the motion of a particle.
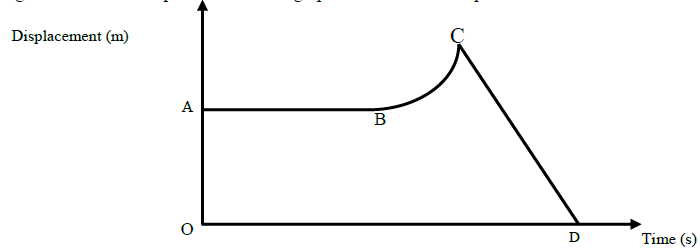
State the nature of the motion of the particle between?
(i) AB
(ii) BC
(iii CD
Date posted:
September 6, 2019
.
Answers (1)
-
A substance of mass 2kg and specific heat capacity 400J/kg/k initially at 800C is immersed in water at 190C. If the final temperature of the...
(Solved)
A substance of mass 2kg and specific heat capacity 400J/kg/k initially at 800C is immersed in water at 190C. If the final temperature of the mixture is 200C. Calculate the mass of water. (Specific heat capacity of water = 4200J/kg/k)
Date posted:
September 6, 2019
.
Answers (1)
-
A force of 20N is used to stretch a spring through 5cm. Calculate the elastic potential energy stored in the spring.
(Solved)
A force of 20N is used to stretch a spring through 5cm. Calculate the elastic potential energy stored in the spring.
Date posted:
September 6, 2019
.
Answers (1)
-
A pipe of radius 3mm is connected to another pipe of radius 9mm. If water flows in the water pipe at a speed of 2m/s,...
(Solved)
A pipe of radius 3mm is connected to another pipe of radius 9mm. If water flows in the water pipe at a speed of 2m/s, what is the speed in the narrower pipe?
Date posted:
September 6, 2019
.
Answers (1)
-
A uniform 120m metal rod is pivoted near one of its ends and kept in equilibrium by a spring balance as shown in the figure...
(Solved)
A uniform 120m metal rod is pivoted near one of its ends and kept in equilibrium by a spring balance as shown in the figure below.
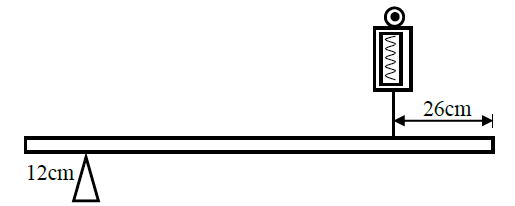
The reading indicated by the spring balance is 2.0N. Work out the mass of the metal rod. (g = 10N/kg)
Date posted:
September 6, 2019
.
Answers (1)
-
The spiral springs shown in the figure below are identical. Each spring has a constant K = 300N/m.
Determine the extension caused by the 150N weight...
(Solved)
The spiral springs shown in the figure below are identical. Each spring has a constant K = 300N/m.
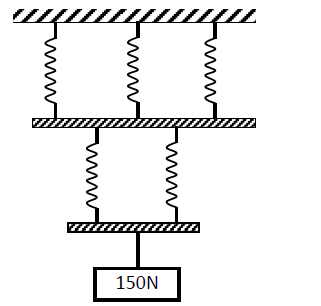
Determine the extension caused by the 150N weight (Ignore weight of springs and connecting rods)
Date posted:
September 6, 2019
.
Answers (1)