- Into a beaker containing dilute nitric acid, add lead oxide until in excess.
- Filter to remove excess lead (II) oxide as residue and lead (II) nitrate as filtrate.
- To filtrate add Na2CO3 solution.
- Filter to remove PbCO3 as residue and sodium nitrate as filtrate.
- Wash residue and dry.
maurice.mutuku answered the question on September 9, 2019 at 05:53
-
Describe the chemical test that can be used to distinguish sodium sulphate from sodium sulphite.
(Solved)
Describe the chemical test that can be used to distinguish sodium sulphate from sodium sulphite.
Date posted:
September 9, 2019
.
Answers (1)
-
(a) State why it's necessary to dry the hydrogen gas before igniting it.
(i) State the precaution that must be taken before igniting the hydrogen.
(Solved)
(a) State why it's necessary to dry the hydrogen gas before igniting it.
(i) State the precaution that must be taken before igniting the hydrogen.
Date posted:
September 6, 2019
.
Answers (1)
-
Hydrogen sulphide gas was bubbled through a solution of zinc nitrate for sometime.
(i) State the observations made.
(ii) Where should the experiment be carried out...
(Solved)
Hydrogen sulphide gas was bubbled through a solution of zinc nitrate for sometime.
(i) State the observations made.
(ii) Where should the experiment be carried out and why?
Date posted:
September 6, 2019
.
Answers (1)
-
The volume of a sample of nitrogen gas at a temperature of 298k and 600 minHg pressures was 4.8 x 10-2m3
Calculate the temperature at which...
(Solved)
The volume of a sample of nitrogen gas at a temperature of 298k and 600 minHg pressures was 4.8 x 10-2m3
Calculate the temperature at which the volume of the gas would be 3.2x102m3 if pressure is constant.
Date posted:
September 6, 2019
.
Answers (1)
-
Ammonia gas is used to manufacture nitric (V) acid as shown below.
(Solved)
Ammonia gas is used to manufacture nitric (V) acid as shown below.
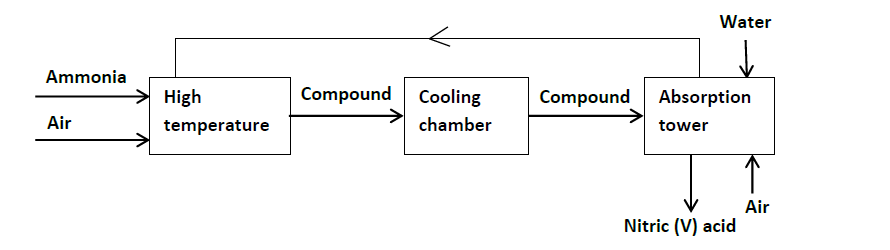
(i) Name the catalyst used in the above process.
(ii) Identify compound U.
(iii) Write the equation for the reaction that took place in the absorption tower.
(iv) Ammonia and nitric (V) acid are used in the manufacture of ammonium nitrate fertilizer, calculate the amount of the fertilizer manufactured per day, if the daily consumption of ammonia is 2400kg. Assume that the factory is 100%
efficient. (N = 14, H = 1, O = 16).
Date posted:
September 6, 2019
.
Answers (1)
-
The diagram below shows an incomplete set-up used to prepare and collect ammonia gas.
(Solved)
The diagram below shows an incomplete set-up used to prepare and collect ammonia gas.
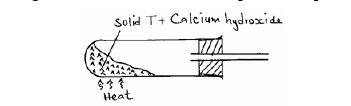
(i) Name solid T.
(ii) Write an equation for the reaction that occurred when a mixture of solid T and calcium hydroxide was heated.
(iii) Complete the diagram to show how a dry sample of ammonia gas can be collected.
Date posted:
September 6, 2019
.
Answers (1)
-
In an experiment to determine the molar heat of reaction when zinc displaces copper, 0.4g of zinc powder were added to 25.0cm³ of 2.0M copper...
(Solved)
In an experiment to determine the molar heat of reaction when zinc displaces copper, 0.4g of zinc powder were added to 25.0cm³ of 2.0M copper (II) sulphate solution. The temperature of copper (II) sulphate solution was 24ºC, while that of the mixture was 36ºC.
(i) Other than increase in temperature, state and explain the observations which were made during the reaction.
(ii) Calculate the heat change during the reaction. (Specific heat capacity of the solution = 4.2Jgˉ¹Kˉ¹ and the density of the solution = 1g/cm³.
(iii) Determine the molar heat of displacement of copper by zinc. (Zn = 65).
Date posted:
September 6, 2019
.
Answers (1)
-
1g of element T was completely converted to its chloride, TCl2 The mass of the chloride formed was 3.96g. Calculate the relative atomic mass of...
(Solved)
1g of element T was completely converted to its chloride, TCl2 The mass of the chloride formed was 3.96g. Calculate the relative atomic mass of element T. (Cl = 35.5).
Date posted:
September 6, 2019
.
Answers (1)
-
Illustrate bonding in carbon (II) oxide using dot and cross (C – 6, O – 8).
(Solved)
Illustrate bonding in carbon (II) oxide using dot and cross (C – 6, O – 8).
Date posted:
September 6, 2019
.
Answers (1)
-
The apparatus shown below were set-up to prepare and collect hydrogen sulphide gas.
(Solved)
The apparatus shown below were set-up to prepare and collect hydrogen sulphide gas.
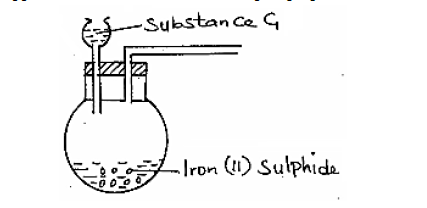
(a) Name substance G.
(b) Complete the set up to show how a dry sample of hydrogen sulphide gas is collected.
Date posted:
September 5, 2019
.
Answers (1)
-
An organic compound Y was analysed and found to contain carbon, hydrogen and oxygen only. 1.29g of Y on complete combustion gave 2.64g of carbon...
(Solved)
An organic compound Y was analysed and found to contain carbon, hydrogen and oxygen only. 1.29g of Y on complete combustion gave 2.64g of carbon (IV) oxide and 0.81g of water. Find the empirical formula of Y. (C = 12, H = 1, O = 16).
Date posted:
September 5, 2019
.
Answers (1)
-
The diagram below shows an experiment for investigating electrical conductivity in lead (II) iodide.
Study it and answer the questions that follow.
(Solved)
The diagram below shows an experiment for investigating electrical conductivity in lead (II) iodide.
Study it and answer the questions that follow.
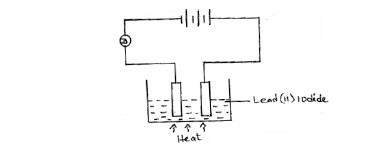
(a) On the diagram;
(i) Label the cathode.
(ii) Show the direction of movement of electrons.
(b) Write an equation for the reaction that takes place at the anode.
Date posted:
September 5, 2019
.
Answers (1)
-
Element X is found in period 3 group (IV) it consists of two isotopes 28X and QX. A sample of X was found to consist...
(Solved)
Element X is found in period 3 group (IV) it consists of two isotopes 28X and QX. A sample of X was found to consist of 90% of 28X if the relative atomic mass of X is 28.3, work out the number of neutrons in QX.
Date posted:
September 5, 2019
.
Answers (1)
-
Study the flow chart below and answer the questions that follow.
(Solved)
Study the flow chart below and answer the questions that follow.
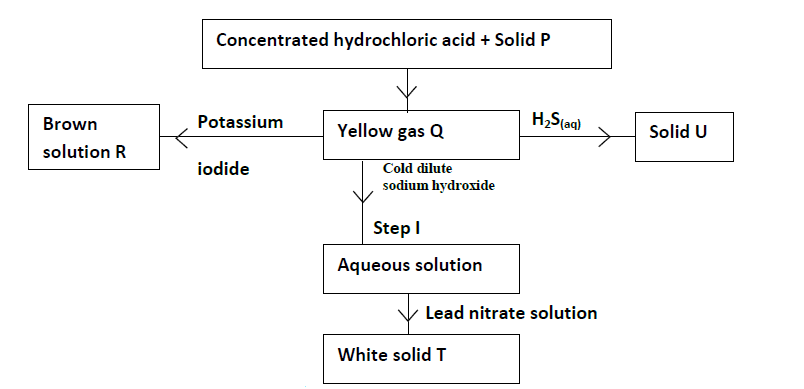
(a) Give the names of the following.
(i) Solid P
(ii) Solid U
(iii) Solid T
(b) Write the equation for the reaction taking place in Step I.
(c) Write the equation for the reaction between concentrated hydrochloric acid and solid P.
(d) Explain what would happen if hot concentrated sodium hydroxide was used in place of cold dilute sodium
hydroxide.
Date posted:
September 5, 2019
.
Answers (1)
-
The diagram below shows an energy level diagram for the formation of magnesium chloride. Study it and answer the questions that follow.
(Solved)
The diagram below shows an energy level diagram for the formation of magnesium chloride. Study it and answer the questions that follow.
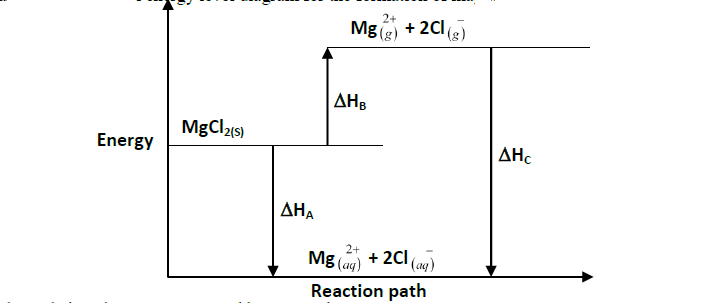
(i) State the enthalpy changes represented by A,B and C
(ii) What is the relationship between 
Date posted:
September 5, 2019
.
Answers (1)
-
Chlorine is used to prepare vinyl chloride (chloroethene) CH2 = CHCl.
(i) State why vinyl chloride (CH2 = CHCl) undergoes addition polymerization.
(ii) Name the polymer...
(Solved)
Chlorine is used to prepare vinyl chloride (chloroethene) CH2 = CHCl.
(i) State why vinyl chloride (CH2 = CHCl) undergoes addition polymerization.
(ii) Name the polymer formed.
(iii) Complete the following equation to show how the two monomers combine during polymerization.
CH2 = CHCl + CH2 = CHCl----->
Date posted:
September 4, 2019
.
Answers (1)
-
State two reasons why tin coating is used in food cans.
(Solved)
State two reasons why tin coating is used in food cans.
Date posted:
September 4, 2019
.
Answers (1)
-
When the oxide of metal Z is heated in the presence of metal X, it is reduced.The oxide of metal X is reduced by metal...
(Solved)
When the oxide of metal Z is heated in the presence of metal X, it is reduced.
The oxide of metal X is reduced by metal Y. Arrange the three metals in order of increasing reactivity.
Date posted:
September 4, 2019
.
Answers (1)
-
Study the information in the table below and answer the questions that follow:
(Solved)
Study the information in the table below and answer the questions that follow:
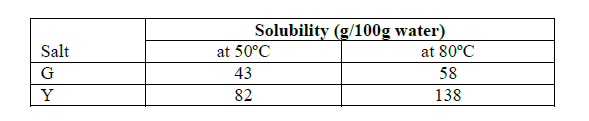
A mixture containing 40g salt G and 120g salt Y in 100g of water at 80ºC was cooled to 50ºC.
(a) Which salt crystallized out? Give reason.
(b) Calculate the mass of the salt that crystallized out.
Date posted:
September 4, 2019
.
Answers (1)
-
Calculate the molar enthalpy of formation of ethyne (C2H2) given the following.
(Solved)
Calculate the molar enthalpy of formation of ethyne (C2H2) given the following.
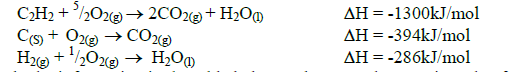
Date posted:
September 4, 2019
.
Answers (1)