(a) - The equilibrium shifts to the right to replace the ammonia gas absorbed by water molecules.
(b) - The equilibrium shifts to the right to use up the nitrogen gas added.
(c) - Forward reaction if exothermic hence favoured by low temperatures.
Therefore the equilibrium shifts to the right.
maurice.mutuku answered the question on September 9, 2019 at 08:13
- Using oxidation numbers, determine from the equation below the species which undergoes oxidation and reduction.
2FeCl2(aq) + Cl2(g)----->2Fe Cl3(aq)
Oxidation -
Reduction -(Solved)
Using oxidation numbers, determine from the equation below the species which undergoes oxidation and reduction.
2FeCl2(aq) + Cl2(g)----->2Fe Cl3(aq)
Oxidation -
Reduction -
Date posted: September 9, 2019. Answers (1)
- Calculate the oxidation state of chromium in the ion Cr2 O2-(Solved)
Calculate the oxidation state of chromium in the ion Cr2 O2-
Date posted: September 9, 2019. Answers (1)
- Gas Q with a relative molecular mass of 48 took 50 seconds to diffuse through a porous diaphragm. How long will it take for the...(Solved)
Gas Q with a relative molecular mass of 48 took 50 seconds to diffuse through a porous diaphragm. How long will it take for the same amount of hydrogen Chloride (HCl) to diffuse through the same diaphragm under similar conditions? (H = 1.0, Cl = 35.5).
Date posted: September 9, 2019. Answers (1)
- Draw a well labeled diagram showing how blister copper is purified.(Solved)
Draw a well labeled diagram showing how blister copper is purified.
Date posted: September 9, 2019. Answers (1)
- The molar heat of fusion of ice at O0C is 6kJ mol-1. Calculate the heat change when 36g of ice is converted to 36g of...(Solved)
The molar heat of fusion of ice at O0C is 6kJ mol-1. Calculate the heat change when 36g of ice is converted to 36g of water at 1O0C. (SHC = 4.2-1g K-1, density = 1.0g/cm3, H = 1.0, O = 16.0)
Date posted: September 9, 2019. Answers (1)
- Name the following organic compounds.(Solved)
I.Name the following organic compounds.
(a) 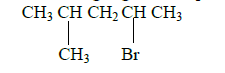
(b) HOCH2 - CHOH - CH2OH
II.Given:
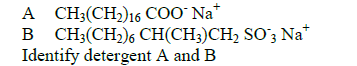
Date posted: September 9, 2019. Answers (1)
- Study the diagram below (Solved)
Study the diagram below
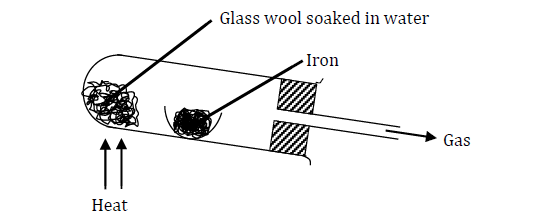
Why would it not be advisable to use potassium in place of iron in the set-up?
Date posted: September 9, 2019. Answers (1)
- 6.95g of hydrated iron (II) sulphate FeSO4. nH2O was dissolved in 250 cm3 solution resulting into a 0.1M solution.
Determine the value of n. (Fe =...(Solved)
6.95g of hydrated iron (II) sulphate FeSO4. nH2O was dissolved in 250 cm3 solution resulting into a 0.1M solution.
Determine the value of n. (Fe = 56, O = 16, S = 32, H = 1).
Date posted: September 9, 2019. Answers (1)
- A, B, C are three homologous series of organic compounds.(Solved)
A, B, C are three homologous series of organic compounds.
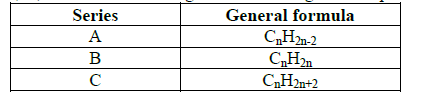
(i) What is the name given to series C?
(ii) Write down the name and structural formula of the second member of series “B”.
(iii) Draw the structural formulae of the first two members of the series "A".
(iv) Complete the balance in the following equation:
CH3CH3 + O2 →
Date posted: September 9, 2019. Answers (1)
- Starting with lead oxide, describe how a pure sample of lead carbonate can be prepared in the laboratory.(Solved)
Starting with lead oxide, describe how a pure sample of lead carbonate can be prepared in the laboratory.
Date posted: September 9, 2019. Answers (1)
- Describe the chemical test that can be used to distinguish sodium sulphate from sodium sulphite.(Solved)
Describe the chemical test that can be used to distinguish sodium sulphate from sodium sulphite.
Date posted: September 9, 2019. Answers (1)
- To determine the heat of combustion of ethanol, a form four class used the set-up shown below.(Solved)
To determine the heat of combustion of ethanol, a form four class used the set-up shown below.

(i) Fill in the missing information in the class table.
(ii) The 50ml of water used is de-ionised and its specific heat capacity c, is 4.2kJ/Kg/K. Calculate heat of combustion of the ethanol used.
(iii) Calculate the moles of ethanol that were burnt (RMM of ethanol = 46).
(iv) Calculate the heat of combustion per mole of ethanol.
Date posted: September 9, 2019. Answers (1)
- The grid below is a Periodic Table. The letters used are not the actual chemical symbols of the elements. Study the table and answer the...(Solved)
The grid below is a Periodic Table. The letters used are not the actual chemical symbols of the elements. Study the table and answer the questions that follow.
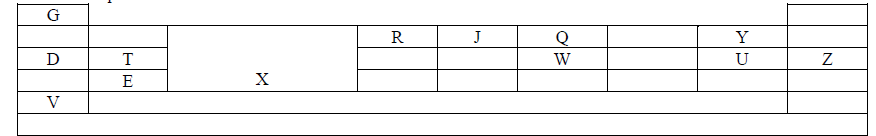
(a) What name is given to the elements that occupy region X?
(b) Write an equation to show how element Y forms an ion.
(c) Compare the atomic and ionic radius of T.
(d) Explain why the ionization energy of V is lower than that of D.
(e) Name the chemical family to which E belongs.
(f) An element A is in period 3 and it loses three electrons to form an ion. Write the electronic configuration of an atom of A.
(g) Elements D and U combine to form a compound with a giant structure.
(i) Name the giant structure.
(ii) State two characteristics of the structure.
(h) Write the formula of the compound formed when T and Y react.
(i) State and explain the change in electrical conductivity from D to T.
Date posted: September 9, 2019. Answers (1)
- The table below gives some physical properties of substances A, B and C. Study it and answer the questions that follow.(Solved)
The table below gives some physical properties of substances A, B and C. Study it and answer the questions that follow.
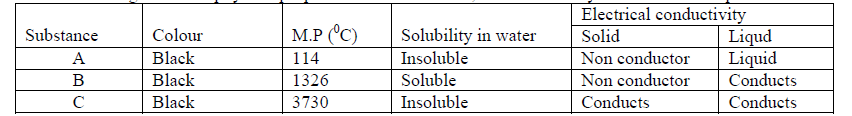
Identify the substance that is:
(i) Giant atomic structure
(ii) Ionic structure
Date posted: September 6, 2019. Answers (1)
- Use the information below to answer the questions that follow:-(Solved)
Use the information below to answer the questions that follow:-
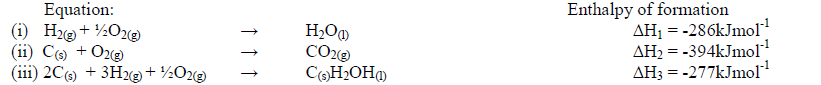
Calculate the molar enthalpy of combustion of ethanol. Given that:
C(s)H2OH(1) + 3O2(g) → 2CO2(g) + 3H2O(1)
Date posted: September 6, 2019. Answers (1)
- In the industrial extraction of lead metal, the ore is first roasted in a furnace. The solid mixture obtained is then fed into another furnace...(Solved)
In the industrial extraction of lead metal, the ore is first roasted in a furnace. The solid mixture obtained is then fed into another furnace together with coke limestone and scrape iron. State the functions of each of the following in this process:-
(a) Coke
(b) Scrape iron
(c) Limestone
Date posted: September 6, 2019. Answers (1)
- (a) State why it's necessary to dry the hydrogen gas before igniting it.
(i) State the precaution that must be taken before igniting the hydrogen.(Solved)
(a) State why it's necessary to dry the hydrogen gas before igniting it.
(i) State the precaution that must be taken before igniting the hydrogen.
Date posted: September 6, 2019. Answers (1)
- Hydrogen sulphide gas was bubbled through a solution of zinc nitrate for sometime.
(i) State the observations made.
(ii) Where should the experiment be carried out...(Solved)
Hydrogen sulphide gas was bubbled through a solution of zinc nitrate for sometime.
(i) State the observations made.
(ii) Where should the experiment be carried out and why?
Date posted: September 6, 2019. Answers (1)
- The volume of a sample of nitrogen gas at a temperature of 298k and 600 minHg pressures was 4.8 x 10-2m3
Calculate the temperature at which...(Solved)
The volume of a sample of nitrogen gas at a temperature of 298k and 600 minHg pressures was 4.8 x 10-2m3
Calculate the temperature at which the volume of the gas would be 3.2x102m3 if pressure is constant.
Date posted: September 6, 2019. Answers (1)
- Ammonia gas is used to manufacture nitric (V) acid as shown below.(Solved)
Ammonia gas is used to manufacture nitric (V) acid as shown below.
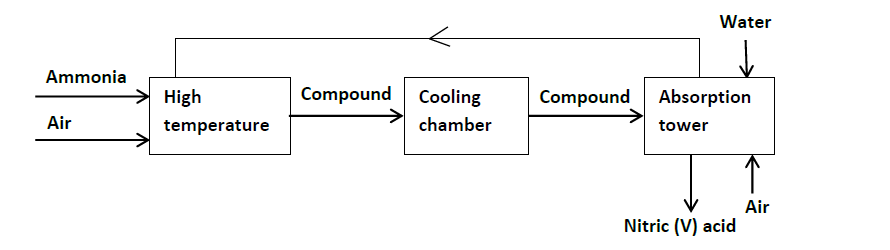
(i) Name the catalyst used in the above process.
(ii) Identify compound U.
(iii) Write the equation for the reaction that took place in the absorption tower.
(iv) Ammonia and nitric (V) acid are used in the manufacture of ammonium nitrate fertilizer, calculate the amount of the fertilizer manufactured per day, if the daily consumption of ammonia is 2400kg. Assume that the factory is 100%
efficient. (N = 14, H = 1, O = 16).
Date posted: September 6, 2019. Answers (1)