-
Given the following. A filter funnel, a thermometer, a stop watch, ice at 00C, an immersion heater rated P watts, a beaker, a stand, boss...
(Solved)
Given the following. A filter funnel, a thermometer, a stop watch, ice at 00C, an immersion heater rated P watts, a beaker, a stand, boss and clamp and weighing machine.Describe an experiment to determine the specific latent heat of fusion of ice. Clearly state the measurements to be made.
Date posted:
September 9, 2019
.
Answers (1)
-
The figure below shows dots which were made by a ticker timer – tape attached to a trolley. The trolley was moving in the direction...
(Solved)
The figure below shows dots which were made by a ticker timer – tape attached to a trolley. The trolley was moving in the direction shown.
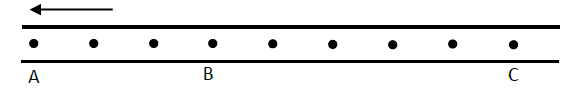
If the frequency used was 60Hz, distance AB = 12cm and BC = 7.2cm, determine
(i) The velocities between AB and BC
(ii) The acceleration of the trolley.
(b) An object is projected horizontally with a velocity of 40m/s at the top of a cliff 100m from the ground. (Take g = 10m/s2)
(i) Calculate the time taken for the object to hit the ground.
(ii) What is the range of the object from the foot of the cliff
Date posted:
September 9, 2019
.
Answers (1)
-
Sketch a block and tackle pulley with three movable pulleys in the lower block and two fixed pulleys in the upper block,to give a velocity...
(Solved)
Sketch a block and tackle pulley with three movable pulleys in the lower block and two fixed pulleys in the upper block,to give a velocity ratio of 6.
Find:
(i) An effort of 450N is used to raise a load of 2700N. Determine:
- Mechanical advantage (M.A)
- Efficiency of the pulley system.
(ii) If all the wasted energy is used to raise the lower block and the frictional force between pulleys and moving parts is 3.6N; determine the weight of the lower block.
(iii) If the load moved through a distance of 50cm, determine the useful work done by the effort.
Date posted:
September 9, 2019
.
Answers (1)
-
The spiral springs shown in the figure below are identical. Each spring has a spring constant K = 200 N/m. Each rod weighs 0.1 N...
(Solved)
The spiral springs shown in the figure below are identical. Each spring has a spring constant K = 200 N/m. Each rod weighs 0.1 N and each spring weigh 0.1 N. Determine the total extension caused by the 150 N weight.
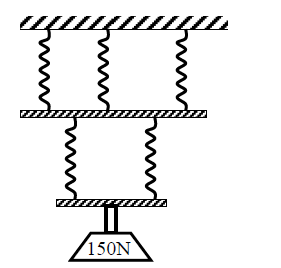
Date posted:
September 9, 2019
.
Answers (1)
-
A balloon with argon gas of volume 199cm3 at the earth‘s surface where the temperature is 210C, and the pressure 760mm of mercury. If it...
(Solved)
A balloon with argon gas of volume 199cm3 at the earth‘s surface where the temperature is 210C, and the pressure 760mm of mercury. If it is allowed to ascend to a height where the temperature is 20C and the pressure 100mm of mercury, calculate the volume of the balloon.
Date posted:
September 9, 2019
.
Answers (1)
-
In a faulty mercury-in-glass thermometer it was found that the mercury level stands at 2 cm mark in the tube at 00C and 20cm when...
(Solved)
In a faulty mercury-in-glass thermometer it was found that the mercury level stands at 2 cm mark in the tube at 00C and 20cm when in steam above boiling point of water at normal atmospheric pressure. Calculate the temperature when the mercury stands at 13cm mark.
Date posted:
September 9, 2019
.
Answers (1)
-
A micrometer screw gauge which had an error of +0.02mm was used to measure the diameter of a spherical marble. If the actual diameter was...
(Solved)
A micrometer screw gauge which had an error of +0.02mm was used to measure the diameter of a spherical marble. If the actual diameter was 3.67mm, draw a micrometer screw gauge showing its reading.
Date posted:
September 9, 2019
.
Answers (1)
-
In an experiment to determine the focal length of a converging lens several values of image distance and the corresponding magnification were obtained. A graph...
(Solved)
In an experiment to determine the focal length of a converging lens several values of image distance and the corresponding magnification were obtained. A graph of magnification m against image distance (V) was plotted as shown below.
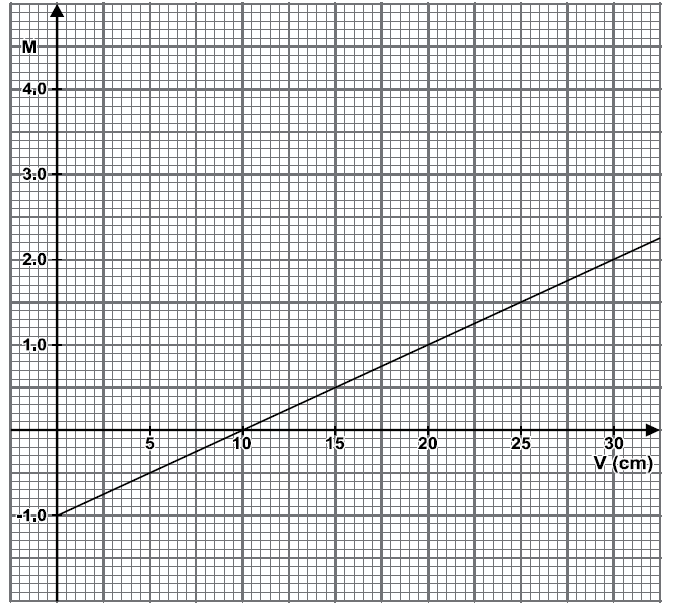
From the graph determine the focal length of the converging lens.
Date posted:
September 9, 2019
.
Answers (1)
-
The figure below represents a pinhole camera.Sketch rays to show the formation of an enlarged image in the camera. Label both the image and the...
(Solved)
The figure below represents a pinhole camera.Sketch rays to show the formation of an enlarged image in the camera. Label both the image and the object.
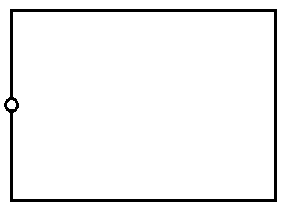
Date posted:
September 9, 2019
.
Answers (1)
-
The figure below shows a bar magnet. Point A and B are in front of the magnet.On the axis provided, sketch a graph showing how...
(Solved)
The figure below shows a bar magnet. Point A and B are in front of the magnet.

On the axis provided, sketch a graph showing how the magnetic field strength changes from A to B.
Date posted:
September 9, 2019
.
Answers (1)
-
The following set-up was used by a student to determine the relative density of a cork
(Solved)
The following set-up was used by a student to determine the relative density of a cork
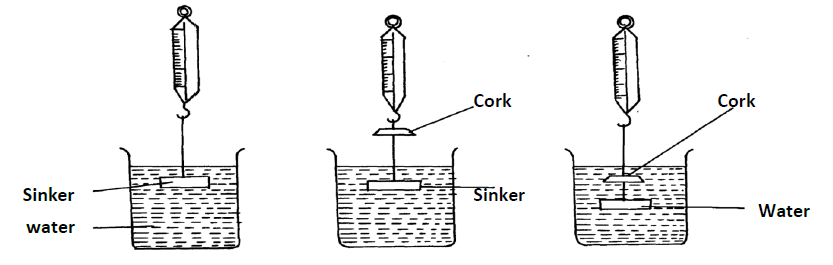
During the experiment, the following measurements were taken:-
- Weight of sinker in water = w1
- Weight of sinker in water and cork in air = w2
- Weight of sinker and cork in water = w3
(i) Write an expression for the up thrust on cork
(ii) Write an expression for the relative density of the cork
Date posted:
September 9, 2019
.
Answers (1)
-
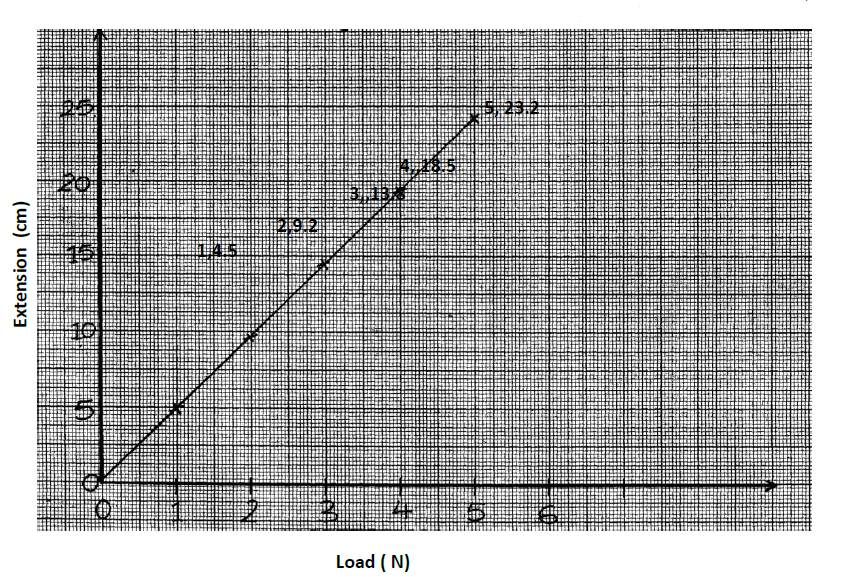
The graph shows how extension e of a helical spring varied with load, hanging on it. (cm).Determine from the graph, the proportionality constant of the...
(Solved)
The graph shows how extension e of a helical spring varied with load, hanging on it. (cm).Determine from the graph, the proportionality constant of the spring.
Date posted:
September 9, 2019
.
Answers (1)
-
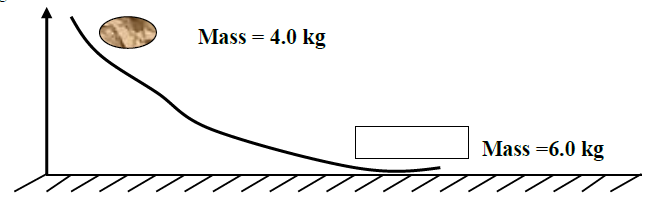
A body of mass 4.0 kg held at a vertical height of 500cm is released to travel along a frictionless curved path as shown in...
(Solved)
A body of mass 4.0 kg held at a vertical height of 500cm is released to travel along a frictionless curved path as shown in the figure below.The 4.0kg mass strikes body of mass 6.0kg at rest immediately it reaches the horizontal. The bodies stick together and move in the same direction. Determine the velocity of the bodies immediately after collision.
Date posted:
September 9, 2019
.
Answers (1)
-
The figure below shows a simple pendulum of length 80 cm. the pendulum bob whose mass is 50 g oscillates between points A and B,...
(Solved)
The figure below shows a simple pendulum of length 80 cm. the pendulum bob whose mass is 50 g oscillates between points A and B, through its rest position C. A and B are both 10 cm higher than C.
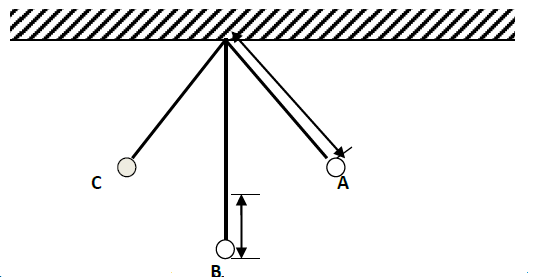
(a) State the form of energy possessed by the pendulum bob at point A
(b) Determine
(i) The velocity of the bob at point C
(ii) The tension in the string as the bob passes point C
(Take acceleration due to gravity g=10m/s2
Date posted:
September 9, 2019
.
Answers (1)
-
The figure below shows an inclined plane and a load of mass 15kg pulled by an effort of 100N.Find the efficiency of the machine.
(Solved)
The figure below shows an inclined plane and a load of mass 15kg pulled by an effort of 100N.Find the efficiency of the machine.
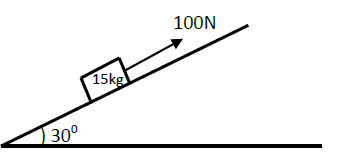
Date posted:
September 9, 2019
.
Answers (1)
-
The figure below shows a U-tube manometer containing a gas, mercury and water. Calculate the gas pressure acting on the mercury. (Take atmospheric pressure to...
(Solved)
The figure below shows a U-tube manometer containing a gas, mercury and water. Calculate the gas pressure acting on the mercury. (Take atmospheric pressure to be 1.05 x 105 pa, density of mercury and water to be 13600kg/m3 and 1000kg/m3 respectively).
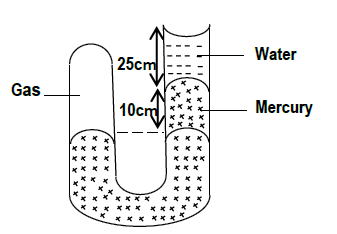
Date posted:
September 9, 2019
.
Answers (1)
-
The figure below shows an electromagnetic relay being used to switch an electric motor on and off. The electromagnet consists of a coil of wire...
(Solved)
The figure below shows an electromagnetic relay being used to switch an electric motor on and off. The electromagnet consists of a coil of wire wrapped around a core. The motor in figure is switched off.
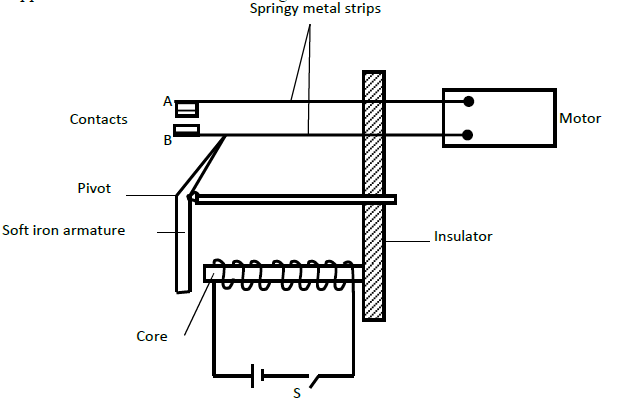
(a) Suggest suitable material for the core.
(b) What happens to the core when switch S is closed?
(c) Why do the contacts A and B close when the switch S is closed.
(d) When the switch S is opened, what will happen to;
(i) The core
(ii) Soft iron armature.
Date posted:
September 9, 2019
.
Answers (1)
-
The figure below shows a circuit with resistors and voltmeter connected to a battery.
(Solved)
The figure below shows a circuit with resistors and voltmeter connected to a battery.
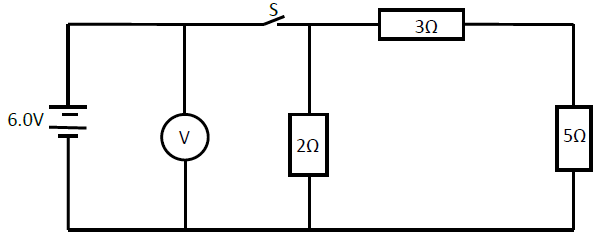
(i) If each cell has an internal resistance of 0.7 ohms, determine the total resistance in the circuit.
(ii) What amount of current flows through the 3 ohms resistor when the switch is closed?
(iii) What is the reading of the voltmeter when the switch S is
(I) Open
(II) Closed
(iv) Account for the difference between the answers in (I) and (II) above.
Date posted:
September 9, 2019
.
Answers (1)
-
A transformer has 200 turns in the primary coil and 1000 turns in the secondary coil. The primary coil is connected to an a.c source...
(Solved)
A transformer has 200 turns in the primary coil and 1000 turns in the secondary coil. The primary coil is connected to an a.c source producing 100 V and rated 500 W. The current delivered by the secondary circuit was found to be 0.95 A.
(i) Determine the efficiency of this transformer.
(ii) Explain why the efficiency is less than 100%.
Date posted:
September 6, 2019
.
Answers (1)
-
A bar magnet is pushed into a coil as shown in the figure below.
(Solved)
A bar magnet is pushed into a coil as shown in the figure below.
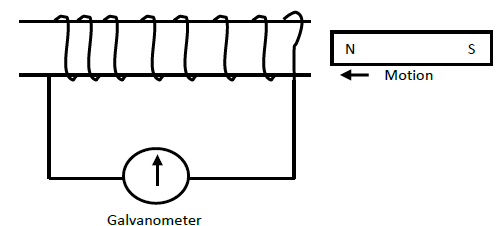
Explain what happens to the pointer of the galvanometer when the magnet is:
(i) Moved into the coil rapidly?
(ii) Remains stationary inside the coil?
Date posted:
September 6, 2019
.
Answers (1)