-
The grid below shows part of the periodic table. Use it to answer the questions that follow. The letters do not represent actual symbols.
(Solved)
The grid below shows part of the periodic table. Use it to answer the questions that follow. The letters do not represent actual symbols.
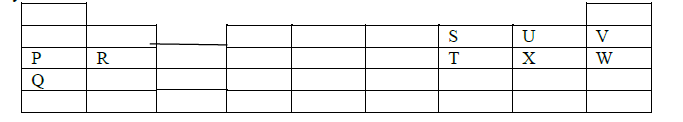
(a) Which of the elements has the highest atomic radius? Explain.
(b) Identify the most reactive Oxidizing agent. Explain.
(c) Compare the atomic radius of P and R. Explain
(d) Give the formula of one stable ion with an electron arrangement of 2.8 which is:
(i) A Negatively charged divalent ion.
(ii) A Positively charged monovalent.
(e) Given that the mass number of W is 40. Write down the composition of its nucleus
(f) Write the formula of the compounds formed between.
(i) Element R and X.
(ii) Give one property of the structure formed when R and X bond.
Date posted:
September 9, 2019
.
Answers (1)
-
Study the following part of the solvay process for the manufacture of sodium carbonate and answer the questions that follow:
(Solved)
Study the following part of the solvay process for the manufacture of sodium carbonate and answer the questions that follow:
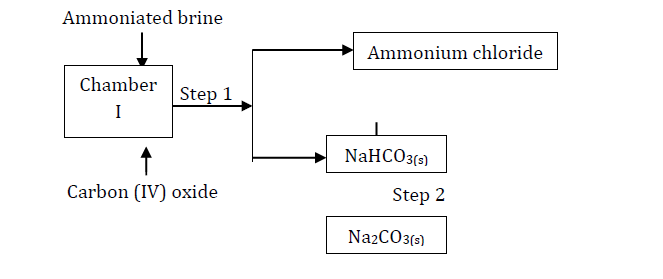
(i) State the main source of Carbon (IV) oxide in the process.
(ii) Write down the overall equation for the reaction in chamber I.
(iii) Name process in step 1.
Date posted:
September 9, 2019
.
Answers (1)
-
Gas Q with a relative molecular mass of 48 took 50 seconds to diffuse through a porous diaphragm. How long will it take for the...
(Solved)
Gas Q with a relative molecular mass of 48 took 50 seconds to diffuse through a porous diaphragm. How long will it take for the same amount of hydrogen Chloride (HCl) to diffuse through the same diaphragm under similar conditions? (H = 1.0, Cl = 35.5).
Date posted:
September 9, 2019
.
Answers (1)
-
Draw a well labeled diagram showing how blister copper is purified.
(Solved)
Draw a well labeled diagram showing how blister copper is purified.
Date posted:
September 9, 2019
.
Answers (1)
-
The molar heat of fusion of ice at O0C is 6kJ mol-1. Calculate the heat change when 36g of ice is converted to 36g of...
(Solved)
The molar heat of fusion of ice at O0C is 6kJ mol-1. Calculate the heat change when 36g of ice is converted to 36g of water at 1O0C. (SHC = 4.2-1g K-1, density = 1.0g/cm3, H = 1.0, O = 16.0)
Date posted:
September 9, 2019
.
Answers (1)
-
6.95g of hydrated iron (II) sulphate FeSO4. nH2O was dissolved in 250 cm3 solution resulting into a 0.1M solution.
Determine the value of n. (Fe =...
(Solved)
6.95g of hydrated iron (II) sulphate FeSO4. nH2O was dissolved in 250 cm3 solution resulting into a 0.1M solution.
Determine the value of n. (Fe = 56, O = 16, S = 32, H = 1).
Date posted:
September 9, 2019
.
Answers (1)
-
Starting with lead oxide, describe how a pure sample of lead carbonate can be prepared in the laboratory.
(Solved)
Starting with lead oxide, describe how a pure sample of lead carbonate can be prepared in the laboratory.
Date posted:
September 9, 2019
.
Answers (1)
-
Describe the chemical test that can be used to distinguish sodium sulphate from sodium sulphite.
(Solved)
Describe the chemical test that can be used to distinguish sodium sulphate from sodium sulphite.
Date posted:
September 9, 2019
.
Answers (1)
-
(a) State why it's necessary to dry the hydrogen gas before igniting it.
(i) State the precaution that must be taken before igniting the hydrogen.
(Solved)
(a) State why it's necessary to dry the hydrogen gas before igniting it.
(i) State the precaution that must be taken before igniting the hydrogen.
Date posted:
September 6, 2019
.
Answers (1)
-
Hydrogen sulphide gas was bubbled through a solution of zinc nitrate for sometime.
(i) State the observations made.
(ii) Where should the experiment be carried out...
(Solved)
Hydrogen sulphide gas was bubbled through a solution of zinc nitrate for sometime.
(i) State the observations made.
(ii) Where should the experiment be carried out and why?
Date posted:
September 6, 2019
.
Answers (1)
-
The volume of a sample of nitrogen gas at a temperature of 298k and 600 minHg pressures was 4.8 x 10-2m3
Calculate the temperature at which...
(Solved)
The volume of a sample of nitrogen gas at a temperature of 298k and 600 minHg pressures was 4.8 x 10-2m3
Calculate the temperature at which the volume of the gas would be 3.2x102m3 if pressure is constant.
Date posted:
September 6, 2019
.
Answers (1)
-
Ammonia gas is used to manufacture nitric (V) acid as shown below.
(Solved)
Ammonia gas is used to manufacture nitric (V) acid as shown below.
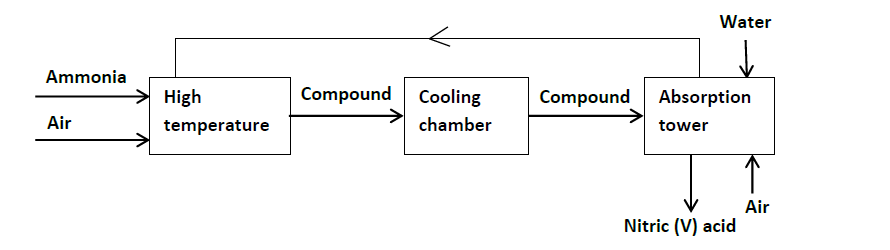
(i) Name the catalyst used in the above process.
(ii) Identify compound U.
(iii) Write the equation for the reaction that took place in the absorption tower.
(iv) Ammonia and nitric (V) acid are used in the manufacture of ammonium nitrate fertilizer, calculate the amount of the fertilizer manufactured per day, if the daily consumption of ammonia is 2400kg. Assume that the factory is 100%
efficient. (N = 14, H = 1, O = 16).
Date posted:
September 6, 2019
.
Answers (1)
-
The diagram below shows an incomplete set-up used to prepare and collect ammonia gas.
(Solved)
The diagram below shows an incomplete set-up used to prepare and collect ammonia gas.
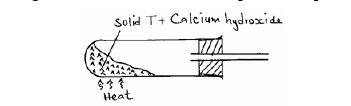
(i) Name solid T.
(ii) Write an equation for the reaction that occurred when a mixture of solid T and calcium hydroxide was heated.
(iii) Complete the diagram to show how a dry sample of ammonia gas can be collected.
Date posted:
September 6, 2019
.
Answers (1)
-
In an experiment to determine the molar heat of reaction when zinc displaces copper, 0.4g of zinc powder were added to 25.0cm³ of 2.0M copper...
(Solved)
In an experiment to determine the molar heat of reaction when zinc displaces copper, 0.4g of zinc powder were added to 25.0cm³ of 2.0M copper (II) sulphate solution. The temperature of copper (II) sulphate solution was 24ºC, while that of the mixture was 36ºC.
(i) Other than increase in temperature, state and explain the observations which were made during the reaction.
(ii) Calculate the heat change during the reaction. (Specific heat capacity of the solution = 4.2Jgˉ¹Kˉ¹ and the density of the solution = 1g/cm³.
(iii) Determine the molar heat of displacement of copper by zinc. (Zn = 65).
Date posted:
September 6, 2019
.
Answers (1)
-
1g of element T was completely converted to its chloride, TCl2 The mass of the chloride formed was 3.96g. Calculate the relative atomic mass of...
(Solved)
1g of element T was completely converted to its chloride, TCl2 The mass of the chloride formed was 3.96g. Calculate the relative atomic mass of element T. (Cl = 35.5).
Date posted:
September 6, 2019
.
Answers (1)
-
Illustrate bonding in carbon (II) oxide using dot and cross (C – 6, O – 8).
(Solved)
Illustrate bonding in carbon (II) oxide using dot and cross (C – 6, O – 8).
Date posted:
September 6, 2019
.
Answers (1)
-
The apparatus shown below were set-up to prepare and collect hydrogen sulphide gas.
(Solved)
The apparatus shown below were set-up to prepare and collect hydrogen sulphide gas.
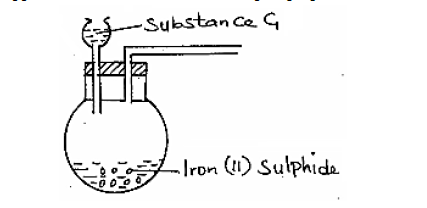
(a) Name substance G.
(b) Complete the set up to show how a dry sample of hydrogen sulphide gas is collected.
Date posted:
September 5, 2019
.
Answers (1)
-
An organic compound Y was analysed and found to contain carbon, hydrogen and oxygen only. 1.29g of Y on complete combustion gave 2.64g of carbon...
(Solved)
An organic compound Y was analysed and found to contain carbon, hydrogen and oxygen only. 1.29g of Y on complete combustion gave 2.64g of carbon (IV) oxide and 0.81g of water. Find the empirical formula of Y. (C = 12, H = 1, O = 16).
Date posted:
September 5, 2019
.
Answers (1)
-
The diagram below shows an experiment for investigating electrical conductivity in lead (II) iodide.
Study it and answer the questions that follow.
(Solved)
The diagram below shows an experiment for investigating electrical conductivity in lead (II) iodide.
Study it and answer the questions that follow.
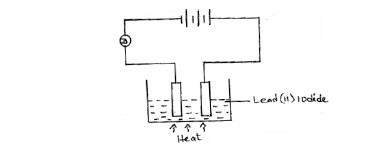
(a) On the diagram;
(i) Label the cathode.
(ii) Show the direction of movement of electrons.
(b) Write an equation for the reaction that takes place at the anode.
Date posted:
September 5, 2019
.
Answers (1)
-
Element X is found in period 3 group (IV) it consists of two isotopes 28X and QX. A sample of X was found to consist...
(Solved)
Element X is found in period 3 group (IV) it consists of two isotopes 28X and QX. A sample of X was found to consist of 90% of 28X if the relative atomic mass of X is 28.3, work out the number of neutrons in QX.
Date posted:
September 5, 2019
.
Answers (1)