-
In a certain reaction, 18.7cm3 of a dibasic acid H2 X required 25cm3 of 0.1M NaOH for complete neutralization.
(a) How many moles of Sodium...
(Solved)
In a certain reaction, 18.7cm3 of a dibasic acid H2 X required 25cm3 of 0.1M NaOH for complete neutralization.
(a) How many moles of Sodium hydroxide are contained in 25cm3?
(b) Calculate the molarity of the dibasic acid.
Date posted:
September 10, 2019
.
Answers (1)
-
The diagram below shows a set-up of apparatus that can be used to prepare nitrogen (IV) oxide. Study it and use it to answer the...
(Solved)
The diagram below shows a set-up of apparatus that can be used to prepare nitrogen (IV) oxide. Study it and use it to answer the questions that follow.
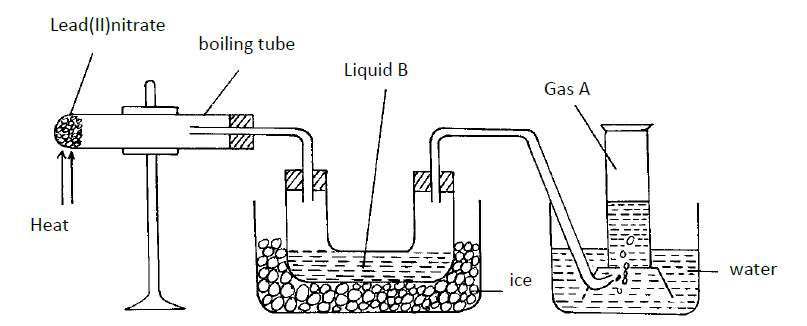
(i) Write the equation for the reaction that takes place in the boiling tube.
(ii) State the observations made in the boiling tube.
(iii) Explain why lead (II) nitrate is preferred over other metal nitrates in this experiment.
(iv) Describe how gas A can be identified.
(b) (i) Name liquid B
(ii) Write a chemical equation to show how liquid B is formed in this experiment.
(c) (i) In another experiment, excess aqueous lead (II) nitrate solution was reacted with a solution which contained 2.34g of sodium chloride. Calculate the mass of precipitate formed in this reaction. (Pb = 207, Cl = 35.5, Na = 23)
(ii) Write an ionic equation for the reaction that takes place when nitrogen (IV) oxide reacts with aqueous sodium hydroxide.
Date posted:
September 10, 2019
.
Answers (1)
-
The flow chart below shows some processes in the extraction of zinc. Study it and answer the questions that follow.
(Solved)
The flow chart below shows some processes in the extraction of zinc. Study it and answer the questions that follow.
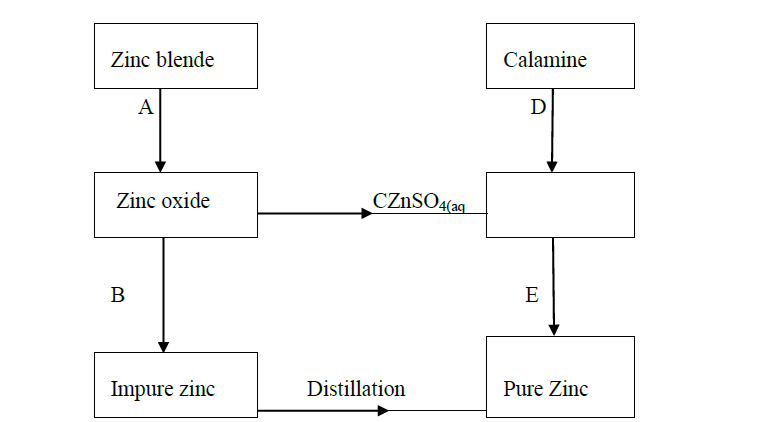
(a) Name the processes represented by A and E.
(b) State the reagents required for processes B, C and D.
(c) Write a chemical equation of the reaction that occurs in process B.
(d) With an aid of a diagram, explain how you would obtain a pure sample of zinc by process E
(e) State two commercial uses of zinc metal.
Date posted:
September 10, 2019
.
Answers (1)
-
The enthalpies of combustion of calcium, carbon and decomposition of calcium carbonate are indicated below;
(Solved)
The enthalpies of combustion of calcium, carbon and decomposition of calcium carbonate are indicated below;
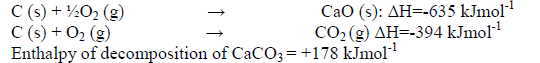
(i) Draw an energy cycle diagram that links the enthalpy of formation of calcium carbonate to enthalpies of combustion of calcium, carbon and decomposition of calcium carbonate.
(ii) Determine the enthalpy of formation of calcium carbonate.
Date posted:
September 10, 2019
.
Answers (1)
-
Study the scheme below and answer the questions that follow.
(Solved)
Study the scheme below and answer the questions that follow.
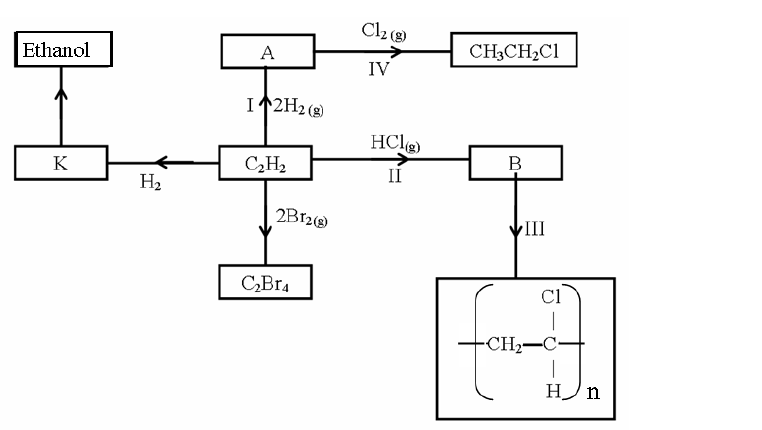
(i) What name is given to the type of cleansing agent prepared by the method above?
(ii) Name one chemical substance added in step II
(iii) What is the purpose of adding the chemical substance named in a (ii) above?
(iv) Name any other suitable substance that can be used in step I
(v) Explain how an aqueous solution of the cleansing agent removes oil during washing
Date posted:
September 10, 2019
.
Answers (1)
-
Determine oxidation number of chlorine
(Solved)
Determine oxidation number of chlorine in 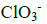
Date posted:
September 10, 2019
.
Answers (1)
-
0.22g of metal Q is deposited by electrolysis when a current of 0.06A flows for 99 minutes.
(RAM of Q = 184, 1F = 96500c)
(Solved)
0.22g of metal Q is deposited by electrolysis when a current of 0.06A flows for 99 minutes.
(RAM of Q = 184, 1F = 96500c)
(i) Find the number of moles of Q deposited.
(ii) Determine the value of n in the metallic ion Qn+
Date posted:
September 10, 2019
.
Answers (1)
-
An aqueous solution of zinc sulphate is electrolysed using platinum electrodes as shown in the set up below.
(Solved)
An aqueous solution of zinc sulphate is electrolysed using platinum electrodes as shown in the set up below.
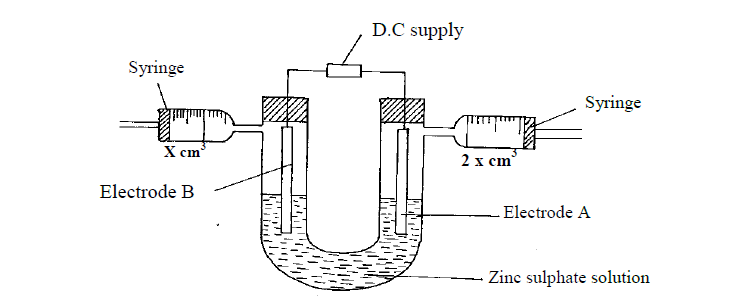
(a) (i) Write a half equation for the reaction taking place at electrode A.
(ii) Identify electrodes A and B
(iii) State and explain the observation at electrode B if copper plate was used instead of platinum electrode.
Date posted:
September 10, 2019
.
Answers (1)
-
The grid below shows part of the periodic table. Use it to answer the questions that follow. The letters do not represent actual symbols.
(Solved)
The grid below shows part of the periodic table. Use it to answer the questions that follow. The letters do not represent actual symbols.
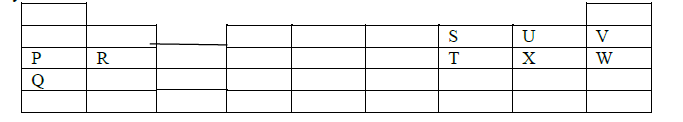
(a) Which of the elements has the highest atomic radius? Explain.
(b) Identify the most reactive Oxidizing agent. Explain.
(c) Compare the atomic radius of P and R. Explain
(d) Give the formula of one stable ion with an electron arrangement of 2.8 which is:
(i) A Negatively charged divalent ion.
(ii) A Positively charged monovalent.
(e) Given that the mass number of W is 40. Write down the composition of its nucleus
(f) Write the formula of the compounds formed between.
(i) Element R and X.
(ii) Give one property of the structure formed when R and X bond.
Date posted:
September 9, 2019
.
Answers (1)
-
Study the section of the polymer below and answer the questions that follow.
(Solved)
Study the section of the polymer below and answer the question that follow.
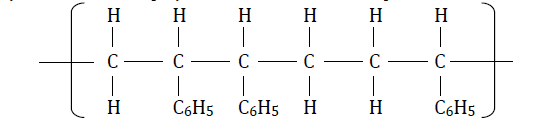
Name the polymer
Date posted:
September 9, 2019
.
Answers (1)
-
An element O has two isotopes..
(Solved)
An element O has two isotopes 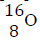 containing 90% and Isotope
containing 90% and Isotope 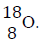
Find the R.A.M of O.
Date posted:
September 9, 2019
.
Answers (1)
-
Study the following part of the solvay process for the manufacture of sodium carbonate and answer the questions that follow:
(Solved)
Study the following part of the solvay process for the manufacture of sodium carbonate and answer the questions that follow:
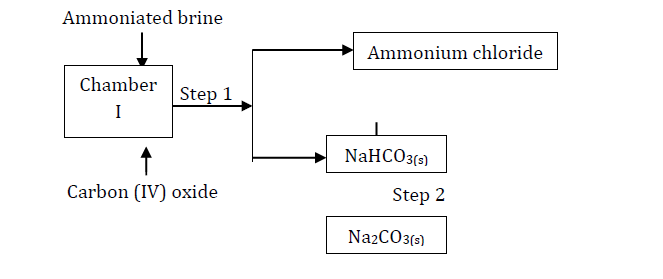
(i) State the main source of Carbon (IV) oxide in the process.
(ii) Write down the overall equation for the reaction in chamber I.
(iii) Name process in step 1.
Date posted:
September 9, 2019
.
Answers (1)
-
What is the atomic number and mass number T?
(Solved)
A radioactive isotope T decays by emitting three alpha particles to form 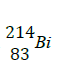 .
.
What is the atomic number and mass number T?
Atomic number -
Mass number -
Date posted:
September 9, 2019
.
Answers (1)
-
Give the name of the following ion [Zn(NH3)4]2+
(Solved)
Give the name of the following ion [Zn(NH3)4]2+
Date posted:
September 9, 2019
.
Answers (1)
-
Write ionic equations to show how excess sodium hydroxide added to a solution containing Al3+ ions reacts.
(Solved)
Write ionic equations to show how excess sodium hydroxide added to a solution containing Al3+ ions reacts.
Date posted:
September 9, 2019
.
Answers (1)
-
Elements P and Q have the following atomic numbers 19 and 8 respectively.
Using dot and cross draw a diagram to show how the elements form...
(Solved)
Elements P and Q have the following atomic numbers 19 and 8 respectively.
Using dot and cross draw a diagram to show how the elements form bonds.
Date posted:
September 9, 2019
.
Answers (1)
-
Given elements A, B and C with atomic numbers 11, 19 and 13 respectively.
(a) Compare the atomic radius of A and C. Explain.
(b) Compare...
(Solved)
Given elements A, B and C with atomic numbers 11, 19 and 13 respectively.
(a) Compare the atomic radius of A and C. Explain.
(b) Compare reactivity of A and B.
Date posted:
September 9, 2019
.
Answers (1)
-
Haber process (the manufacture of ammonia gas) is given by the following equation.
(Solved)
Haber process (the manufacture of ammonia gas) is given by the following equation.
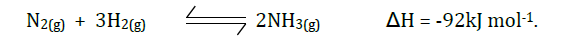
State and explain the effect of:
(a) Introducing some drops of water to the equilibrium.
(b) Pumping nitrogen gas to the equilibrium mixture.
(c) Lowering the temperature of the reaction.
Date posted:
September 9, 2019
.
Answers (1)
-
Calculate the oxidation state of chromium in the ion Cr2 O2-
(Solved)
Calculate the oxidation state of chromium in the ion Cr2 O2-
Date posted:
September 9, 2019
.
Answers (1)
-
Gas Q with a relative molecular mass of 48 took 50 seconds to diffuse through a porous diaphragm. How long will it take for the...
(Solved)
Gas Q with a relative molecular mass of 48 took 50 seconds to diffuse through a porous diaphragm. How long will it take for the same amount of hydrogen Chloride (HCl) to diffuse through the same diaphragm under similar conditions? (H = 1.0, Cl = 35.5).
Date posted:
September 9, 2019
.
Answers (1)