- The figure shows a wheel and axle being used to raise a load W by applying an effort F. the radius of the large wheel...(Solved)
The figure shows a wheel and axle being used to raise a load W by applying an effort F. the radius of the large wheel is R and of the small wheel r as shown.
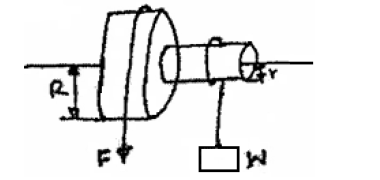
(i) Shows that the velocity ratio (V.R) of this machine is given by R/r.
(ii) Given that r = 5cm, R = 8cm, determine effort required to raise a load of 20N if the efficiency of the machine is 80%.
(iii) It is observed that the efficiency of the machines increases when it is used to lift large loads. Give a reason for this.
Date posted: September 10, 2019. Answers (1)
- Draw a single pulley arrangement with a velocity ratio of 2.(Solved)
Draw a single pulley arrangement with a velocity ratio of 2.
Date posted: September 10, 2019. Answers (1)
- A ball of mass 0.2kg is thrown vertically upwards with velocity of 8ms¯¹. The air resistance is 0.5N. Determine:
(i) the resultant force on the ball...(Solved)
A ball of mass 0.2kg is thrown vertically upwards with velocity of 8msˉ¹. The air resistance is 0.5N. Determine:
(i) the resultant force on the ball as it moves up;
(Take acceleration due to gravity g = 10msˉ²).
(ii) The acceleration of the ball.
(iii) The maximum height reached by the ball.
Date posted: September 10, 2019. Answers (1)
- A hot air balloon falling through the air attains terminal velocity after a short-time. State the reason why it attains terminal velocity.(Solved)
A hot air balloon falling through the air attains terminal velocity after a short-time. State the reason why it attains terminal velocity.
Date posted: September 10, 2019. Answers (1)
- A steel ball of mass 0.05kg was placed on top of a spring on a level ground. The spring was then compressed through a distance...(Solved)
A steel ball of mass 0.05kg was placed on top of a spring on a level ground. The spring was then compressed through a distance of 0.2m.If the spring constant is 15N/m. Calculate the maximum height reached when the spring is released.
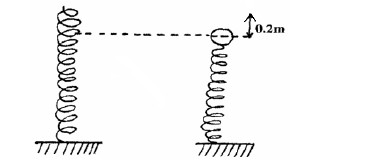
Date posted: September 10, 2019. Answers (1)
- The figure below shows a long tube filled with water. The open end is then covered with a cardboard and tube is inverted. It is...(Solved)
The figure below shows a long tube filled with water. The open end is then covered with a cardboard and tube is inverted. It is observed that the water in the tube does not spill out.Explain the observation.
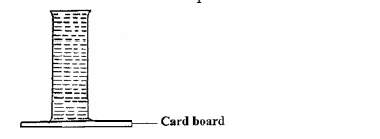
Date posted: September 10, 2019. Answers (1)
- The figure below is a uniform bar of length 2.0m pivoted near one end. The bar is balanced horizontal by a spring.(Solved)
The figure below is a uniform bar of length 2.0 m pivoted near one end. The bar is balanced horizontal by a spring.Given that the tension on the spring is 1.2N, determine the weight of the bar.
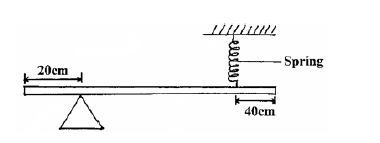
Date posted: September 10, 2019. Answers (1)
- A constant force is applied to a body moving with a constant speed. State one observable change in the state of motion of the body...(Solved)
A constant force is applied to a body moving with a constant speed. State one observable change in the state of motion of the body likely to occur?
Date posted: September 10, 2019. Answers (1)
- The figure below shows a ball projected horizontally.(Solved)
The figure below shows a ball projected horizontally.
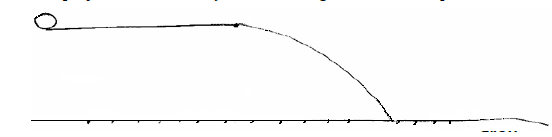
A player taps the ball and makes it spin in anticlockwise direction as it moves.
a. Show the new path followed by the ball.
b. Explain how the ball attains the new path above.
Date posted: September 10, 2019. Answers (1)
- Figure below shows a graph of how the vertical height through which a machine raises a mass 30kg varies with time.(Solved)
Figure below shows a graph of how the vertical height through which a machine raises a mass 30kg varies with time.
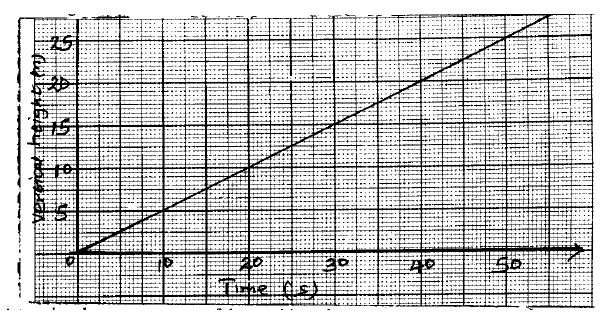
Determine the power output of the machine after 40 seconds.
Date posted: September 10, 2019. Answers (1)
- The reading on a mercury barometer at a place is 690mm. The barometer contains some air which exerts a pressure of 15Nm¯². What is the...(Solved)
The reading on a mercury barometer at a place is 690mm. The barometer contains some air which exerts a pressure of 15Nm¯². What is the pressure at the place Nm¯². (Density of mercury is 1.36 x 104kgm¯³).
Date posted: September 10, 2019. Answers (1)
- The figure below shows part of micrometer screw gauge with 50 divisions on the thimble scale. Complete the diagram to show a reading of 5.73mm.(Solved)
The figure below shows part of micrometer screw gauge with 50 divisions on the thimble scale. Complete the diagram to show a reading of 5.73mm.
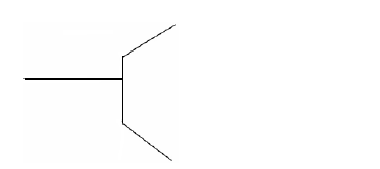
Date posted: September 10, 2019. Answers (1)
- The figure below shows a measuring cylinder,which contains water initially at level A. A solid of mass 0.32g is immersed in the water,the level rises...(Solved)
The figure below shows a measuring cylinder,which contains water initially at level A. A solid of mass 0.32g is immersed in the water,the level rises to B.
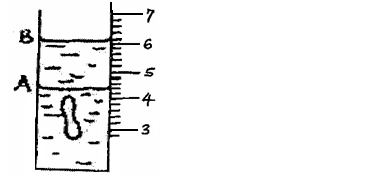
Determine the density of the solid. (Give your answer to 3 significant figures).
Date posted: September 10, 2019. Answers (1)
- The tube of a CRO is coated with graphite. State three functions of the graphite coating(Solved)
The tube of a CRO is coated with graphite. State three functions of the graphite coating
Date posted: September 10, 2019. Answers (1)
- A signal with a frequency of 50Hz is applied across the Y-plates. If the time base with a period of 0.04s is applied across the...(Solved)
A signal with a frequency of 50Hz is applied across the Y-plates. If the time base with a period of 0.04s is applied across the X-plates, sketch a graph of p.d against time showing the number of waves that can be seen on the screen of the C.R.O
Date posted: September 10, 2019. Answers (1)
- Distinguish between cathode rays and light rays.(Solved)
Distinguish between cathode rays and light rays.
Date posted: September 10, 2019. Answers (1)
- The figure below show the displacement time graph of a wave traveling at 400cm/s.(Solved)
The figure below show the displacement time graph of a wave traveling at 400cm/s.
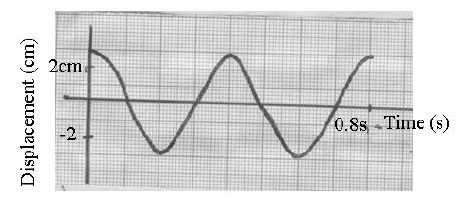
Determine for the wave the:
(i) Amplitude
(ii) Period
(iii) Frequency
(iv) Wavelength
Date posted: September 10, 2019. Answers (1)
- A cooker rated 2.0kW was operated for 40minutes each for 30days. If the cost of each kilo – watt – hour unit is Shs. 15.50,...(Solved)
A cooker rated 2.0kW was operated for 40minutes each for 30days. If the cost of each kilo – watt – hour unit is Shs. 15.50, Calculate the cost of electricity used.
Date posted: September 10, 2019. Answers (1)
- A wire of length 1.5m offers resistance of 6.5 ohms to the flow of current through it. If the cross section area is 5.0×10-6m2.calculate the...(Solved)
A wire of length 1.5m offers resistance of 6.5 ohms to the flow of current through it. If the cross section area is 5.0×10-6m2.calculate the resistivity of the material.
Date posted: September 10, 2019. Answers (1)
- A hunter standing some distance from a cliff blows a whistle and hears its echo 2 seconds later. How far is the cliff from the...(Solved)
A hunter standing some distance from a cliff blows a whistle and hears its echo 2 seconds later. How far is the cliff from the hunter? (speed of sound in air=340m/s)
Date posted: September 10, 2019. Answers (1)