- The grid below represents part of the periodic table. Study it and answer the questions that follow. The letters do not represent the actual symbols...(Solved)
The grid below represents part of the periodic table. Study it and answer the questions that follow. The letters do not represent the actual symbols of the element.

a) What name is given to the group of elements to which Q and R belong?
b) Write the formula of the compound formed when Q and P combine.
c) Name the type of bond formed in (b) above.
d) How does the atomic radii of O and P compare? Give a reason.
e) Draw a dot (.) and cross (x) diagram for the compound formed between N and F.
f) Explain how you would obtain a pure sample of the carbonate of K from its mixture with Lead carbonate powder.
g) Give one use of element M.
h) The melting point of M is -189oC lower than that of F -102oC. Explain this difference in their melting points.
Date posted: September 11, 2019. Answers (1)
- A fixed mass of gas occupies 105cm3 at -140C and 650mmHg pressure. At what temperature will it have a volume of 15cm3 if the pressure...(Solved)
A fixed mass of gas occupies 105cm3 at -140C and 650mmHg pressure. At what temperature will it have a volume of 15cm3 if the pressure is adjusted to 690 mmHg pressure
Date posted: September 11, 2019. Answers (1)
- Below is part of the flow diagram of the contact process.(Solved)
Below is part of the flow diagram of the contact process.

(a) Identify (i) Liquid M
(ii) Liquid N
(b) Write the equation for the reaction taking place in chamber B.
Date posted: September 11, 2019. Answers (1)
- A compound contains only carbon, hydrogen and oxygen .Combustion of 1.068g of the compound produces 1.601g of carbon (IV) oxide and 0.437g of water. The...(Solved)
A compound contains only carbon, hydrogen and oxygen .Combustion of 1.068g of the compound produces 1.601g of carbon (IV) oxide and 0.437g of water. The molar mass of the compound is 176.1 ⁄ . What is the empirical and molecular formulae of the compound?
Date posted: September 10, 2019. Answers (1)
- Give a reason why the formula mass of NO2 is sometimes 92 instead of 46.(Solved)
Give a reason why the formula mass of NO2 is sometimes 92 instead of 46.
Date posted: September 10, 2019. Answers (1)
- Consider the chromatogram below.(Solved)
Consider the chromatogram below.

A piece of chromatogram paper was spotted with colour inks obtained from pens labeled A to F. The diagram above shows the spots after the chromatograph was developed.
(a) Which two pens contained the same pigment?
(b) According to the chromatogram which pigments are present in the inks of the pen number F
(c) Describe how one could get a sample of yellow pigment
Date posted: September 10, 2019. Answers (1)
- Hydrazine gas, shown below, burns in oxygen to form nitrogen gas and steam.(Solved)
Hydrazine gas, shown below, burns in oxygen to form nitrogen gas and steam.

(a) Write an equation for the reaction
(b) Using the bond energies given below, calculate the enthalpy change for the reaction in (a) above

Date posted: September 10, 2019. Answers (1)
- Z grammes of a radioactive isotope take 100 days to decay to 20gms. If the half – life of the element is 25 days.
Calculate the...(Solved)
Z grammes of a radioactive isotope take 100 days to decay to 20gms. If the half – life of the element is 25 days.
Calculate the initial mass of Z of the radio- isotope.
Date posted: September 10, 2019. Answers (1)
- The table below shows the pH values of solutions J to N(Solved)
The table below shows the pH values of solutions J to N

(a) Which solution contains the largest concentration of hydroxide ions?
(b) Which solution is likely to be a solution of acetic acid?
Date posted: September 10, 2019. Answers (1)
- Complete the following equation(Solved)
Complete the following equation
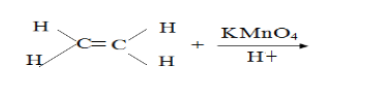
Date posted: September 10, 2019. Answers (1)
- A form one class carried out an experiment to determine the active part of air. The diagram below shows the set-up of the experiment and...(Solved)
A form one class carried out an experiment to determine the active part of air. The diagram below shows the set-up of the experiment and also the observation made.

(a) Identify substance M
(b) State two reasons for the suitability of substance M for this experiment
(c) Write the equation for the reaction of substance M and the active part of air
Date posted: September 10, 2019. Answers (1)
- Draw a dot and cross diagram to show bonding in sulphur (IV) oxide(Solved)
Draw a dot and cross diagram to show bonding in sulphur (IV) oxide
Date posted: September 10, 2019. Answers (1)
- Give one use of charcoal in the sugar refinery industry.(Solved)
Give one use of charcoal in the sugar refinery industry.
Date posted: September 10, 2019. Answers (1)
- Study the flow chart below and answer the questions that follow.(Solved)
Study the flow chart below and answer the questions that follow.

(a) Identify solid G
(b) Write a balanced chemical equation between the yellow solid and dilute nitric acid.
(c) Write the formula of the complex ion in solution F
Date posted: September 10, 2019. Answers (1)
- In a certain reaction, 18.7cm3 of a dibasic acid H2 X required 25cm3 of 0.1M NaOH for complete neutralization.
(a) How many moles of Sodium...(Solved)
In a certain reaction, 18.7cm3 of a dibasic acid H2 X required 25cm3 of 0.1M NaOH for complete neutralization.
(a) How many moles of Sodium hydroxide are contained in 25cm3?
(b) Calculate the molarity of the dibasic acid.
Date posted: September 10, 2019. Answers (1)
- The diagram below shows a set-up of apparatus that can be used to prepare nitrogen (IV) oxide. Study it and use it to answer the...(Solved)
The diagram below shows a set-up of apparatus that can be used to prepare nitrogen (IV) oxide. Study it and use it to answer the questions that follow.
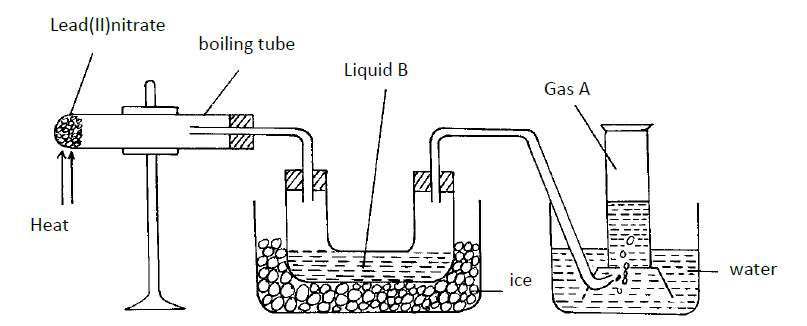
(i) Write the equation for the reaction that takes place in the boiling tube.
(ii) State the observations made in the boiling tube.
(iii) Explain why lead (II) nitrate is preferred over other metal nitrates in this experiment.
(iv) Describe how gas A can be identified.
(b) (i) Name liquid B
(ii) Write a chemical equation to show how liquid B is formed in this experiment.
(c) (i) In another experiment, excess aqueous lead (II) nitrate solution was reacted with a solution which contained 2.34g of sodium chloride. Calculate the mass of precipitate formed in this reaction. (Pb = 207, Cl = 35.5, Na = 23)
(ii) Write an ionic equation for the reaction that takes place when nitrogen (IV) oxide reacts with aqueous sodium hydroxide.
Date posted: September 10, 2019. Answers (1)
- The flow chart below shows some processes in the extraction of zinc. Study it and answer the questions that follow.(Solved)
The flow chart below shows some processes in the extraction of zinc. Study it and answer the questions that follow.
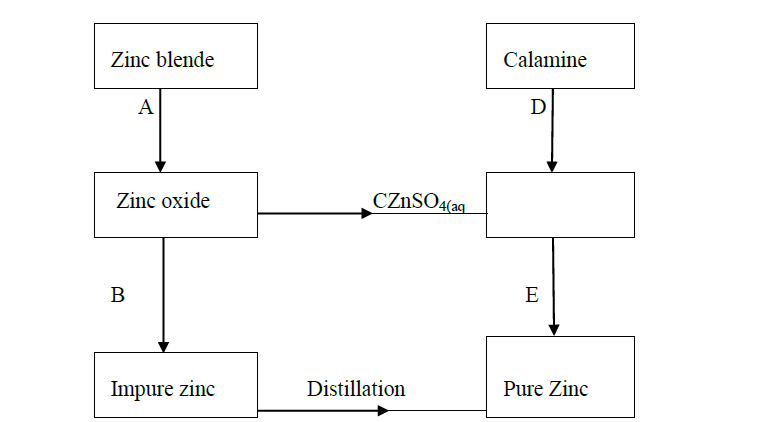
(a) Name the processes represented by A and E.
(b) State the reagents required for processes B, C and D.
(c) Write a chemical equation of the reaction that occurs in process B.
(d) With an aid of a diagram, explain how you would obtain a pure sample of zinc by process E
(e) State two commercial uses of zinc metal.
Date posted: September 10, 2019. Answers (1)
- The enthalpies of combustion of calcium, carbon and decomposition of calcium carbonate are indicated below;(Solved)
The enthalpies of combustion of calcium, carbon and decomposition of calcium carbonate are indicated below;
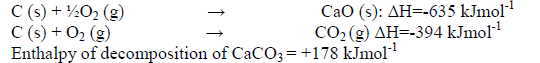
(i) Draw an energy cycle diagram that links the enthalpy of formation of calcium carbonate to enthalpies of combustion of calcium, carbon and decomposition of calcium carbonate.
(ii) Determine the enthalpy of formation of calcium carbonate.
Date posted: September 10, 2019. Answers (1)
- Study the scheme below and answer the questions that follow.(Solved)
Study the scheme below and answer the questions that follow.
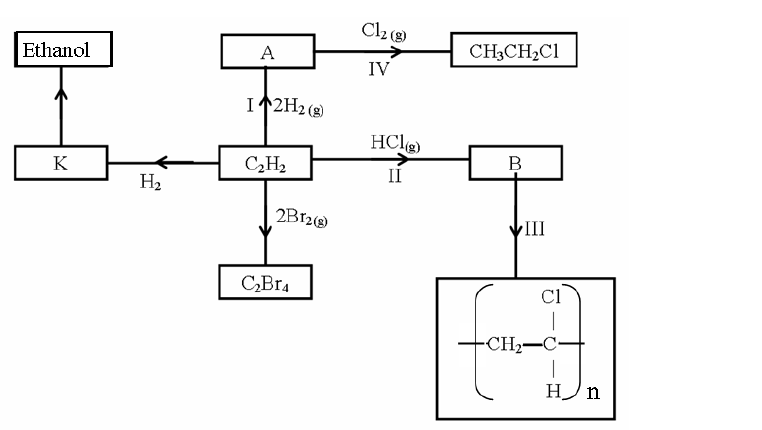
(i) What name is given to the type of cleansing agent prepared by the method above?
(ii) Name one chemical substance added in step II
(iii) What is the purpose of adding the chemical substance named in a (ii) above?
(iv) Name any other suitable substance that can be used in step I
(v) Explain how an aqueous solution of the cleansing agent removes oil during washing
Date posted: September 10, 2019. Answers (1)
- Determine oxidation number of chlorine(Solved)
Determine oxidation number of chlorine in 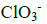
Date posted: September 10, 2019. Answers (1)