-
The enthalpies of combustion of carbon, hydrogen and ethanol are given below.
(Solved)
The enthalpies of combustion of carbon, hydrogen and ethanol are given below.
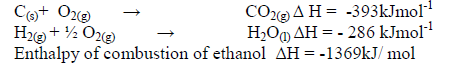
i) Draw an energy cycle diagram that links the enthalpy of formation of ethanol to enthalpies of combustion of Carbon,hydrogen and ethanol.
ii) Determine the enthalpy of formation of ethanol
Date posted:
September 11, 2019
.
Answers (1)
-
A fixed mass of gas occupies 105cm3 at -140C and 650mmHg pressure. At what temperature will it have a volume of 15cm3 if the pressure...
(Solved)
A fixed mass of gas occupies 105cm3 at -140C and 650mmHg pressure. At what temperature will it have a volume of 15cm3 if the pressure is adjusted to 690 mmHg pressure
Date posted:
September 11, 2019
.
Answers (1)
-
Give a reason why the formula mass of NO2 is sometimes 92 instead of 46.
(Solved)
Give a reason why the formula mass of NO2 is sometimes 92 instead of 46.
Date posted:
September 10, 2019
.
Answers (1)
-
Consider the chromatogram below.
(Solved)
Consider the chromatogram below.

A piece of chromatogram paper was spotted with colour inks obtained from pens labeled A to F. The diagram above shows the spots after the chromatograph was developed.
(a) Which two pens contained the same pigment?
(b) According to the chromatogram which pigments are present in the inks of the pen number F
(c) Describe how one could get a sample of yellow pigment
Date posted:
September 10, 2019
.
Answers (1)
-
Hydrazine gas, shown below, burns in oxygen to form nitrogen gas and steam.
(Solved)
Hydrazine gas, shown below, burns in oxygen to form nitrogen gas and steam.

(a) Write an equation for the reaction
(b) Using the bond energies given below, calculate the enthalpy change for the reaction in (a) above

Date posted:
September 10, 2019
.
Answers (1)
-
A form one class carried out an experiment to determine the active part of air. The diagram below shows the set-up of the experiment and...
(Solved)
A form one class carried out an experiment to determine the active part of air. The diagram below shows the set-up of the experiment and also the observation made.

(a) Identify substance M
(b) State two reasons for the suitability of substance M for this experiment
(c) Write the equation for the reaction of substance M and the active part of air
Date posted:
September 10, 2019
.
Answers (1)
-
Draw a dot and cross diagram to show bonding in sulphur (IV) oxide
(Solved)
Draw a dot and cross diagram to show bonding in sulphur (IV) oxide
Date posted:
September 10, 2019
.
Answers (1)
-
Give one use of charcoal in the sugar refinery industry.
(Solved)
Give one use of charcoal in the sugar refinery industry.
Date posted:
September 10, 2019
.
Answers (1)
-
Study the flow chart below and answer the questions that follow.
(Solved)
Study the flow chart below and answer the questions that follow.

(a) Identify solid G
(b) Write a balanced chemical equation between the yellow solid and dilute nitric acid.
(c) Write the formula of the complex ion in solution F
Date posted:
September 10, 2019
.
Answers (1)
-
The diagram below shows a set-up of apparatus that can be used to prepare nitrogen (IV) oxide. Study it and use it to answer the...
(Solved)
The diagram below shows a set-up of apparatus that can be used to prepare nitrogen (IV) oxide. Study it and use it to answer the questions that follow.
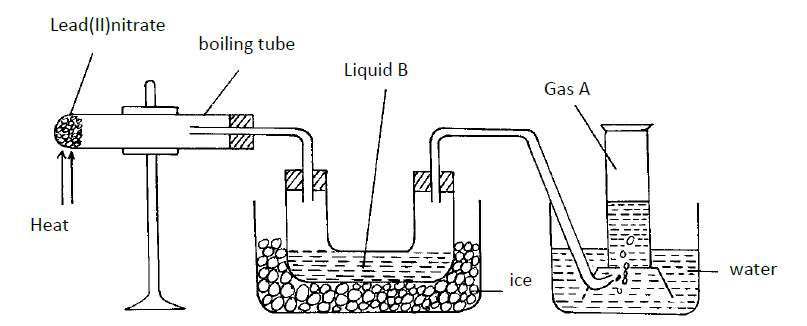
(i) Write the equation for the reaction that takes place in the boiling tube.
(ii) State the observations made in the boiling tube.
(iii) Explain why lead (II) nitrate is preferred over other metal nitrates in this experiment.
(iv) Describe how gas A can be identified.
(b) (i) Name liquid B
(ii) Write a chemical equation to show how liquid B is formed in this experiment.
(c) (i) In another experiment, excess aqueous lead (II) nitrate solution was reacted with a solution which contained 2.34g of sodium chloride. Calculate the mass of precipitate formed in this reaction. (Pb = 207, Cl = 35.5, Na = 23)
(ii) Write an ionic equation for the reaction that takes place when nitrogen (IV) oxide reacts with aqueous sodium hydroxide.
Date posted:
September 10, 2019
.
Answers (1)
-
The flow chart below shows some processes in the extraction of zinc. Study it and answer the questions that follow.
(Solved)
The flow chart below shows some processes in the extraction of zinc. Study it and answer the questions that follow.
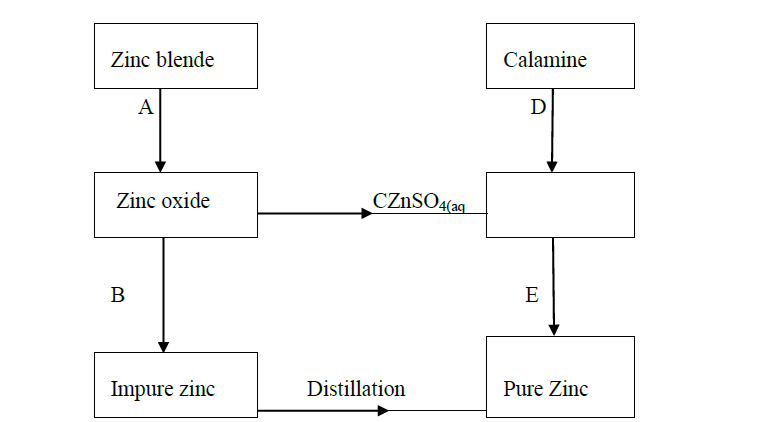
(a) Name the processes represented by A and E.
(b) State the reagents required for processes B, C and D.
(c) Write a chemical equation of the reaction that occurs in process B.
(d) With an aid of a diagram, explain how you would obtain a pure sample of zinc by process E
(e) State two commercial uses of zinc metal.
Date posted:
September 10, 2019
.
Answers (1)
-
The enthalpies of combustion of calcium, carbon and decomposition of calcium carbonate are indicated below;
(Solved)
The enthalpies of combustion of calcium, carbon and decomposition of calcium carbonate are indicated below;
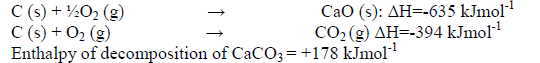
(i) Draw an energy cycle diagram that links the enthalpy of formation of calcium carbonate to enthalpies of combustion of calcium, carbon and decomposition of calcium carbonate.
(ii) Determine the enthalpy of formation of calcium carbonate.
Date posted:
September 10, 2019
.
Answers (1)
-
An aqueous solution of zinc sulphate is electrolysed using platinum electrodes as shown in the set up below.
(Solved)
An aqueous solution of zinc sulphate is electrolysed using platinum electrodes as shown in the set up below.
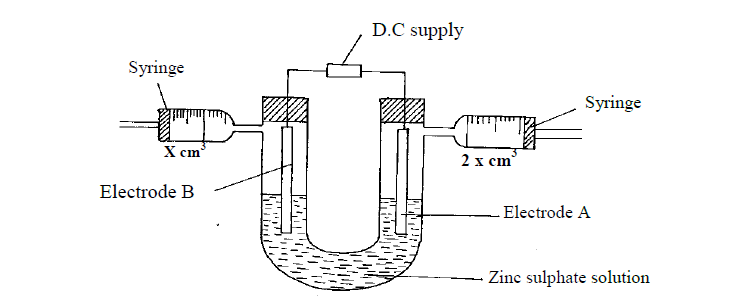
(a) (i) Write a half equation for the reaction taking place at electrode A.
(ii) Identify electrodes A and B
(iii) State and explain the observation at electrode B if copper plate was used instead of platinum electrode.
Date posted:
September 10, 2019
.
Answers (1)
-
The grid below shows part of the periodic table. Use it to answer the questions that follow. The letters do not represent actual symbols.
(Solved)
The grid below shows part of the periodic table. Use it to answer the questions that follow. The letters do not represent actual symbols.
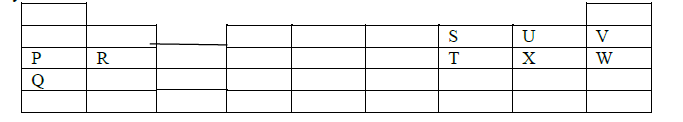
(a) Which of the elements has the highest atomic radius? Explain.
(b) Identify the most reactive Oxidizing agent. Explain.
(c) Compare the atomic radius of P and R. Explain
(d) Give the formula of one stable ion with an electron arrangement of 2.8 which is:
(i) A Negatively charged divalent ion.
(ii) A Positively charged monovalent.
(e) Given that the mass number of W is 40. Write down the composition of its nucleus
(f) Write the formula of the compounds formed between.
(i) Element R and X.
(ii) Give one property of the structure formed when R and X bond.
Date posted:
September 9, 2019
.
Answers (1)
-
Study the following part of the solvay process for the manufacture of sodium carbonate and answer the questions that follow:
(Solved)
Study the following part of the solvay process for the manufacture of sodium carbonate and answer the questions that follow:
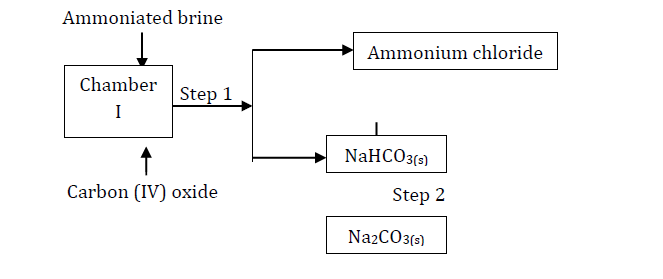
(i) State the main source of Carbon (IV) oxide in the process.
(ii) Write down the overall equation for the reaction in chamber I.
(iii) Name process in step 1.
Date posted:
September 9, 2019
.
Answers (1)
-
Gas Q with a relative molecular mass of 48 took 50 seconds to diffuse through a porous diaphragm. How long will it take for the...
(Solved)
Gas Q with a relative molecular mass of 48 took 50 seconds to diffuse through a porous diaphragm. How long will it take for the same amount of hydrogen Chloride (HCl) to diffuse through the same diaphragm under similar conditions? (H = 1.0, Cl = 35.5).
Date posted:
September 9, 2019
.
Answers (1)
-
Draw a well labeled diagram showing how blister copper is purified.
(Solved)
Draw a well labeled diagram showing how blister copper is purified.
Date posted:
September 9, 2019
.
Answers (1)
-
The molar heat of fusion of ice at O0C is 6kJ mol-1. Calculate the heat change when 36g of ice is converted to 36g of...
(Solved)
The molar heat of fusion of ice at O0C is 6kJ mol-1. Calculate the heat change when 36g of ice is converted to 36g of water at 1O0C. (SHC = 4.2-1g K-1, density = 1.0g/cm3, H = 1.0, O = 16.0)
Date posted:
September 9, 2019
.
Answers (1)
-
6.95g of hydrated iron (II) sulphate FeSO4. nH2O was dissolved in 250 cm3 solution resulting into a 0.1M solution.
Determine the value of n. (Fe =...
(Solved)
6.95g of hydrated iron (II) sulphate FeSO4. nH2O was dissolved in 250 cm3 solution resulting into a 0.1M solution.
Determine the value of n. (Fe = 56, O = 16, S = 32, H = 1).
Date posted:
September 9, 2019
.
Answers (1)
-
Starting with lead oxide, describe how a pure sample of lead carbonate can be prepared in the laboratory.
(Solved)
Starting with lead oxide, describe how a pure sample of lead carbonate can be prepared in the laboratory.
Date posted:
September 9, 2019
.
Answers (1)