- During electrolysis of a sulphate of metal M, using M electrodes, a current of 0.5A was passed through the solution for 12 minutes, 52 seconds....(Solved)
During electrolysis of a sulphate of metal M, using M electrodes, a current of 0.5A was passed through the solution for 12 minutes, 52 seconds. The mass at the cathode increases from 2.40g to 2.44g. Determine the charge of metal M (1f = 96500C,M = 20).
Date posted: September 11, 2019. Answers (1)
- Ammonia gas can be prepared as shown:(Solved)
Ammonia gas can be prepared as shown:

State and explain the observations made if the pressure is increased in the system.
Date posted: September 11, 2019. Answers (1)
- Study the table below and answer the questions that follow.(Solved)
Study the table below and answer the questions that follow.

(a) Write the formula of the chloride of element K.
(b) With reason(s) compare the melting point of the chlorides of element K to that of element L
Date posted: September 11, 2019. Answers (1)
- Xg of sodium hydroxide were dissolved in distilled water to make 100cm3 of solution 40cm3 of this solution required 25cm3 of 1M sulphuric (VI) acid....(Solved)
Xg of sodium hydroxide were dissolved in distilled water to make 100cm3 of solution 40cm3 of this solution required 25cm3 of 1M sulphuric (VI) acid. Determine the mass of X of solution of sodium hydroxide (Na = 23, O = 16, H = 1)
Date posted: September 11, 2019. Answers (1)
- A section of a polymer has the following structure.(Solved)
A section of a polymer has the following structure.

A sample of this polymer is found to have a molecular mass of 1500.
Determine the number of monomers in the polymer (H = 1, C = 12, Cl = 35.5)
Date posted: September 11, 2019. Answers (1)
- Calculate the enthalpy of formation of propane.(Solved)
Given that:
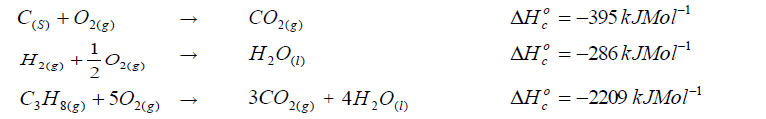
Calculate the enthalpy of formation of propane.
Date posted: September 11, 2019. Answers (1)
- The following set-up was used to prepare Carbon (II) Oxide in laboratory.(Solved)
The following set-up was used to prepare Carbon (II) Oxide in laboratory.

(a) Name liquid X and state its value.
(b) Explain why the gas is collected over water.
Date posted: September 11, 2019. Answers (1)
- Give the name of the following organic compound.(Solved)
Give the name of the following organic compound.
(a) 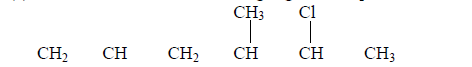
(b) 1 mole of HBr reacts with propene molecules. Draw the structure and name the compound formed.
Date posted: September 11, 2019. Answers (1)
- When 75cm3 of a gaseous hydro-carbon CxHy burns in 250cm3 of oxygen,25cm3 of oxygen is unused, 150cm3 of carbon (IV) oxide is formed. Determine the...(Solved)
When 75cm3 of a gaseous hydro-carbon CxHy burns in 250cm3 of oxygen,25cm3 of oxygen is unused, 150cm3 of carbon (IV) oxide is formed. Determine the volume of steam formed hence deduce the formular of the Hydro-carbon.
Date posted: September 11, 2019. Answers (1)
- Using relevant equations show that zinc oxide is an amphoteric oxide.(Solved)
Using relevant equations show that zinc oxide is an amphoteric oxide.
Date posted: September 11, 2019. Answers (1)
- An isotope M has 20 neutrons and a mass number of 37.(Solved)
An isotope M has 20 neutrons and a mass number of 37.
(i) Draw the atomic structure of M.
(ii) To which group does M belong? Explain.
Date posted: September 11, 2019. Answers (1)
- The diagram below represents a flame of the Bunsen burner.(Solved)
The diagram below represents a flame of the Bunsen burner.

A piece of paper is flipped over the flame as shown in the diagram. Draw a sketch to show the outcome.
Date posted: September 11, 2019. Answers (1)
- The chart below represents the extraction of iron and some of its uses.(Solved)
The chart below represents the extraction of iron and some of its uses.


(a) Name the raw materials fed into the blast furnace.
(b) Name 3 exhaust gases emitted from the blast furnace.
(c) (i) Why is it necessary to convert pig iron into wrought iron
(ii) State one commercial use of iron.
(d) Name substances A,B,C,X,Y
(e) i) Write equations for reactions in steps II and II
ii) Write an ionic equation for the reaction in step I.
iii) What observations are made in steps I and II?
Date posted: September 11, 2019. Answers (1)
- Experiment was set as shown below.(Solved)
Experiment was set as shown below.
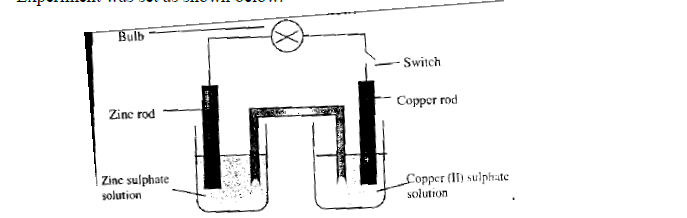
(a) What is observed on the bulb when the switch is closed?
(b) Which electrode will be cathode?
(c) Write down the half-cell equations for:
(i) Copper electrode.
(ii) Zinc electrode.
(d) Write the overall ionic equation for the electrochemical cell.
(e) The table below shows the electrode potentials.
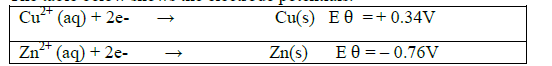
What is the value of the voltage of the cell?
(f) The switch is kept closed. State and explain the observation expected after sometime on the
(i) The zinc rod.
(ii) Copper (II) Sulphate solution.
Date posted: September 11, 2019. Answers (1)
- The enthalpies of combustion of carbon, hydrogen and ethanol are given below.(Solved)
The enthalpies of combustion of carbon, hydrogen and ethanol are given below.
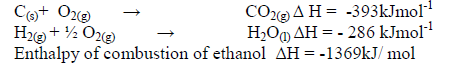
i) Draw an energy cycle diagram that links the enthalpy of formation of ethanol to enthalpies of combustion of Carbon,hydrogen and ethanol.
ii) Determine the enthalpy of formation of ethanol
Date posted: September 11, 2019. Answers (1)
- The list below shows the formulae of some organic compounds. Use letters T1 to T6 to answer the questions that follow.(Solved)
The list below shows the formulae of some organic compounds. Use letters T1 to T6 to answer the questions that follow.
T1 – CH3CH2CH2CH2CH3
T2 – CH3CH2CH2COOC2H5
T3 – CH3CH2CH2CH2OH
T4– CH3CH2CH2COOH
T5 – CH3CH2CHCH2
T6 – CH3CCCH3
(a) Select two compounds which:
(i) Are not hydrocarbons
(ii) Would decolourise both bromine water and acidified potassium manganite (VII)
(iii) Would produce hydrogen gas when reacted with potassium metal
(b) Select a compound which would produce bubbles of a gas when reacted with sodium carbonate.
(c) (i) Identify the compound that is likely to undergo polymerization. Give a reason for your answer. Using two molecules show how polymerization occurs.
I. Compound
II. Reasons
III. Polymerization
IV Name the process by which compound T2 is formed and identify the compounds that were used to form it.
I. Process
II.Compounds
(d) Compound T3 can be converted to T4 as shown by the equation below:
C4H9OH(l) + O2(g)----->C3H7COOH(aq) + H2O(l)
Given the following information:
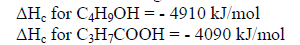
Determine the heat change for the reaction above.
Date posted: September 11, 2019. Answers (1)
- The grid below represents part of the periodic table. Study it and answer the questions that follow. The letters do not represent the actual symbols...(Solved)
The grid below represents part of the periodic table. Study it and answer the questions that follow. The letters do not represent the actual symbols of the element.

a) What name is given to the group of elements to which Q and R belong?
b) Write the formula of the compound formed when Q and P combine.
c) Name the type of bond formed in (b) above.
d) How does the atomic radii of O and P compare? Give a reason.
e) Draw a dot (.) and cross (x) diagram for the compound formed between N and F.
f) Explain how you would obtain a pure sample of the carbonate of K from its mixture with Lead carbonate powder.
g) Give one use of element M.
h) The melting point of M is -189oC lower than that of F -102oC. Explain this difference in their melting points.
Date posted: September 11, 2019. Answers (1)
- A fixed mass of gas occupies 105cm3 at -140C and 650mmHg pressure. At what temperature will it have a volume of 15cm3 if the pressure...(Solved)
A fixed mass of gas occupies 105cm3 at -140C and 650mmHg pressure. At what temperature will it have a volume of 15cm3 if the pressure is adjusted to 690 mmHg pressure
Date posted: September 11, 2019. Answers (1)
- Below is part of the flow diagram of the contact process.(Solved)
Below is part of the flow diagram of the contact process.

(a) Identify (i) Liquid M
(ii) Liquid N
(b) Write the equation for the reaction taking place in chamber B.
Date posted: September 11, 2019. Answers (1)
- A compound contains only carbon, hydrogen and oxygen .Combustion of 1.068g of the compound produces 1.601g of carbon (IV) oxide and 0.437g of water. The...(Solved)
A compound contains only carbon, hydrogen and oxygen .Combustion of 1.068g of the compound produces 1.601g of carbon (IV) oxide and 0.437g of water. The molar mass of the compound is 176.1 ⁄ . What is the empirical and molecular formulae of the compound?
Date posted: September 10, 2019. Answers (1)