- Use standard electrode potentials of elements A, B, C, D and F given below to answer the questions that follow. The letters do not represent...(Solved)
Use standard electrode potentials of elements A, B, C, D and F given below to answer the questions that follow. The letters do not represent the actual symbols of the elements.

(i) Which element is likely to be hydrogen? Give a reason for your answer.
(ii) What is the  value of the strongest reducing agent?
value of the strongest reducing agent?
(iii) In the space provided, draw a labeled diagram of the electrochemical cell that would be obtained when half-cells of element B and D are combined.
(iv) Calculate  the value of the electrochemical cell constructed in (iii) above.
the value of the electrochemical cell constructed in (iii) above.
Date posted: September 12, 2019. Answers (1)
- Study the flow chart below and answer the question that follow.(Solved)
Study the flow chart below and answer the question that follow.

Name the substances: C and D
Date posted: September 12, 2019. Answers (1)
- When 0.1g of a certain compound Q was burned and used to heat 150cm3 of water, the temperature rose from 250C to 290C.
Calculate the relative...(Solved)
When 0.1g of a certain compound Q was burned and used to heat 150cm3 of water, the temperature rose from 250C to 290C.
Calculate the relative molecular mass of the compound given that 1 mole of the compound has an enthalpy of -1072kJ/mol.
(S.h.C = 4.2J Kg-1k-1, density of water = 1g/cm3)
Date posted: September 12, 2019. Answers (1)
- The diagram below shows heating curve of water in the laboratory.(Solved)
The diagram below shows heating curve of water in the laboratory.

(i) At what temperature does the water boil?
(ii) Giving reason(s) is this pure or impure water?
(iii) State the effect of impurities on the boiling point of water?
Date posted: September 12, 2019. Answers (1)
- The curves below represent the volume of carbon (IV) oxide gas evolved when 1M concentrated hydrochloric acid and 60g of calcium carbonate and also 0.5M...(Solved)
The curves below represent the volume of carbon (IV) oxide gas evolved when 1M concentrated hydrochloric acid and 60g of calcium carbonate and also 0.5M concentrated hydrochloric acid was reacted with the same quantity of the carbonate.

(a) Which of the curves represent the reaction of 1M concentrated hydrochloric acid with powdered calcium carbonate.
Explain.
(b) Explain why the curves flatters at the same level of production of carbon (IV) oxide.
Date posted: September 12, 2019. Answers (1)
- Study the diagram and use it to answer the question that follow.(Solved)
Study the diagram and use it to answer the question that follow.

(i) State the observations in the combustion tube.
(ii) Describe the chemical test for product B.
Date posted: September 11, 2019. Answers (1)
- Complete the table below.(Solved)
Complete the table below.

Date posted: September 11, 2019. Answers (1)
- Nitrogen (II) oxide can be prepared by catalytic oxidation of ammonia. Name the catalyst used in the process.(Solved)
Nitrogen (II) oxide can be prepared by catalytic oxidation of ammonia. Name the catalyst used in the process.
Date posted: September 11, 2019. Answers (1)
- When hydrocarbon was completely burnt in air, 2.51g of carbon (IV) oxide and 1.28g of water were formed. Given that the compound has a relative...(Solved)
When hydrocarbon was completely burnt in air, 2.51g of carbon (IV) oxide and 1.28g of water were formed. Given that the compound has a relative molecular mass of 58, find its molecular formular (C = 12, H=1).
Date posted: September 11, 2019. Answers (1)
- During electrolysis of a sulphate of metal M, using M electrodes, a current of 0.5A was passed through the solution for 12 minutes, 52 seconds....(Solved)
During electrolysis of a sulphate of metal M, using M electrodes, a current of 0.5A was passed through the solution for 12 minutes, 52 seconds. The mass at the cathode increases from 2.40g to 2.44g. Determine the charge of metal M (1f = 96500C,M = 20).
Date posted: September 11, 2019. Answers (1)
- Ammonia gas can be prepared as shown:(Solved)
Ammonia gas can be prepared as shown:

State and explain the observations made if the pressure is increased in the system.
Date posted: September 11, 2019. Answers (1)
- Study the table below and answer the questions that follow.(Solved)
Study the table below and answer the questions that follow.

(a) Write the formula of the chloride of element K.
(b) With reason(s) compare the melting point of the chlorides of element K to that of element L
Date posted: September 11, 2019. Answers (1)
- Xg of sodium hydroxide were dissolved in distilled water to make 100cm3 of solution 40cm3 of this solution required 25cm3 of 1M sulphuric (VI) acid....(Solved)
Xg of sodium hydroxide were dissolved in distilled water to make 100cm3 of solution 40cm3 of this solution required 25cm3 of 1M sulphuric (VI) acid. Determine the mass of X of solution of sodium hydroxide (Na = 23, O = 16, H = 1)
Date posted: September 11, 2019. Answers (1)
- A section of a polymer has the following structure.(Solved)
A section of a polymer has the following structure.

A sample of this polymer is found to have a molecular mass of 1500.
Determine the number of monomers in the polymer (H = 1, C = 12, Cl = 35.5)
Date posted: September 11, 2019. Answers (1)
- Calculate the enthalpy of formation of propane.(Solved)
Given that:
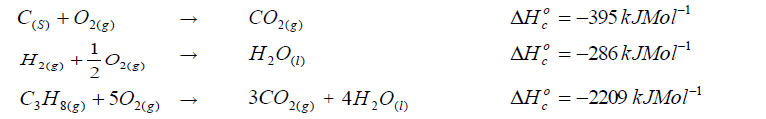
Calculate the enthalpy of formation of propane.
Date posted: September 11, 2019. Answers (1)
- The following set-up was used to prepare Carbon (II) Oxide in laboratory.(Solved)
The following set-up was used to prepare Carbon (II) Oxide in laboratory.

(a) Name liquid X and state its value.
(b) Explain why the gas is collected over water.
Date posted: September 11, 2019. Answers (1)
- Give the name of the following organic compound.(Solved)
Give the name of the following organic compound.
(a) 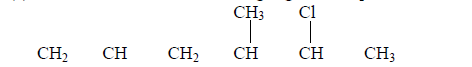
(b) 1 mole of HBr reacts with propene molecules. Draw the structure and name the compound formed.
Date posted: September 11, 2019. Answers (1)
- When 75cm3 of a gaseous hydro-carbon CxHy burns in 250cm3 of oxygen,25cm3 of oxygen is unused, 150cm3 of carbon (IV) oxide is formed. Determine the...(Solved)
When 75cm3 of a gaseous hydro-carbon CxHy burns in 250cm3 of oxygen,25cm3 of oxygen is unused, 150cm3 of carbon (IV) oxide is formed. Determine the volume of steam formed hence deduce the formular of the Hydro-carbon.
Date posted: September 11, 2019. Answers (1)
- Using relevant equations show that zinc oxide is an amphoteric oxide.(Solved)
Using relevant equations show that zinc oxide is an amphoteric oxide.
Date posted: September 11, 2019. Answers (1)
- An isotope M has 20 neutrons and a mass number of 37.(Solved)
An isotope M has 20 neutrons and a mass number of 37.
(i) Draw the atomic structure of M.
(ii) To which group does M belong? Explain.
Date posted: September 11, 2019. Answers (1)