-
Study the following part of periodic table chart and use it to answer the question that follow. The letters are of the actual symbols of...
(Solved)
Study the following part of periodic table chart and use it to answer the question that follow. The letters are of the actual symbols of the elements.

(i) Which elements form ions with charge of -2. Explain.
(ii) Compare the ionic radius of C and E. Explain.
(iii) Write the formular of the compound formed between element G and H.
(iv) In terms of structure of bonding, explain why the oxides of D has a lower melting point than that of G.
(v) Write an equation to show the action of heat on nitrates of F and G.
Date posted:
September 12, 2019
.
Answers (1)
-
The reaction between Lead (II) Nitrate and concentrated sulphuric (VI) acid starts but stops immediately.Explain.
(Solved)
The reaction between Lead (II) Nitrate and concentrated sulphuric (VI) acid starts but stops immediately.Explain.
Date posted:
September 12, 2019
.
Answers (1)
-
The table below gives the solubilities of potassium nitrate at different temperatures.
(Solved)
The table below gives the solubilities of potassium nitrate at different temperatures.

(i) Plot a graph of the solubility of potassium nitrate (vertical axis) against temperature.
(ii) Using the graph:
I. Determine the solubility of potassium nitrate at 150C.
II. Determine the mass of potassium nitrate that remained undissolved given that 80g of potassium nitrate were added to 100cm3 of water and warmed to 400C. (2mks)
(iii) Determine the molar concentration of potassium nitrate at 1500C. (Assume there is no change in density of water at this
temperature) (K = 39.0; N = 14.0; O = 16.0)
Date posted:
September 12, 2019
.
Answers (1)
-
Draw the structural formula of Butyne
(Solved)
Draw the structural formula of Butyne
Date posted:
September 12, 2019
.
Answers (1)
-
The diagram below shows heating curve of water in the laboratory.
(Solved)
The diagram below shows heating curve of water in the laboratory.

(i) At what temperature does the water boil?
(ii) Giving reason(s) is this pure or impure water?
(iii) State the effect of impurities on the boiling point of water?
Date posted:
September 12, 2019
.
Answers (1)
-
The curves below represent the volume of carbon (IV) oxide gas evolved when 1M concentrated hydrochloric acid and 60g of calcium carbonate and also 0.5M...
(Solved)
The curves below represent the volume of carbon (IV) oxide gas evolved when 1M concentrated hydrochloric acid and 60g of calcium carbonate and also 0.5M concentrated hydrochloric acid was reacted with the same quantity of the carbonate.

(a) Which of the curves represent the reaction of 1M concentrated hydrochloric acid with powdered calcium carbonate.
Explain.
(b) Explain why the curves flatters at the same level of production of carbon (IV) oxide.
Date posted:
September 12, 2019
.
Answers (1)
-
Xg of sodium hydroxide were dissolved in distilled water to make 100cm3 of solution 40cm3 of this solution required 25cm3 of 1M sulphuric (VI) acid....
(Solved)
Xg of sodium hydroxide were dissolved in distilled water to make 100cm3 of solution 40cm3 of this solution required 25cm3 of 1M sulphuric (VI) acid. Determine the mass of X of solution of sodium hydroxide (Na = 23, O = 16, H = 1)
Date posted:
September 11, 2019
.
Answers (1)
-
Calculate the enthalpy of formation of propane.
(Solved)
Given that:
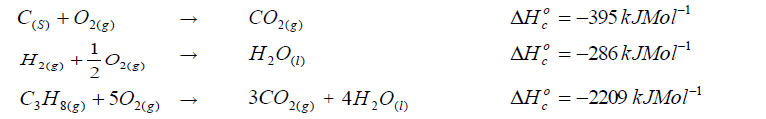
Calculate the enthalpy of formation of propane.
Date posted:
September 11, 2019
.
Answers (1)
-
The following set-up was used to prepare Carbon (II) Oxide in laboratory.
(Solved)
The following set-up was used to prepare Carbon (II) Oxide in laboratory.

(a) Name liquid X and state its value.
(b) Explain why the gas is collected over water.
Date posted:
September 11, 2019
.
Answers (1)
-
Using relevant equations show that zinc oxide is an amphoteric oxide.
(Solved)
Using relevant equations show that zinc oxide is an amphoteric oxide.
Date posted:
September 11, 2019
.
Answers (1)
-
The diagram below represents a flame of the Bunsen burner.
(Solved)
The diagram below represents a flame of the Bunsen burner.

A piece of paper is flipped over the flame as shown in the diagram. Draw a sketch to show the outcome.
Date posted:
September 11, 2019
.
Answers (1)
-
The chart below represents the extraction of iron and some of its uses.
(Solved)
The chart below represents the extraction of iron and some of its uses.


(a) Name the raw materials fed into the blast furnace.
(b) Name 3 exhaust gases emitted from the blast furnace.
(c) (i) Why is it necessary to convert pig iron into wrought iron
(ii) State one commercial use of iron.
(d) Name substances A,B,C,X,Y
(e) i) Write equations for reactions in steps II and II
ii) Write an ionic equation for the reaction in step I.
iii) What observations are made in steps I and II?
Date posted:
September 11, 2019
.
Answers (1)
-
The enthalpies of combustion of carbon, hydrogen and ethanol are given below.
(Solved)
The enthalpies of combustion of carbon, hydrogen and ethanol are given below.
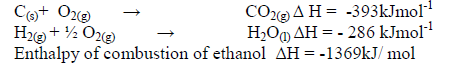
i) Draw an energy cycle diagram that links the enthalpy of formation of ethanol to enthalpies of combustion of Carbon,hydrogen and ethanol.
ii) Determine the enthalpy of formation of ethanol
Date posted:
September 11, 2019
.
Answers (1)
-
A fixed mass of gas occupies 105cm3 at -140C and 650mmHg pressure. At what temperature will it have a volume of 15cm3 if the pressure...
(Solved)
A fixed mass of gas occupies 105cm3 at -140C and 650mmHg pressure. At what temperature will it have a volume of 15cm3 if the pressure is adjusted to 690 mmHg pressure
Date posted:
September 11, 2019
.
Answers (1)
-
Give a reason why the formula mass of NO2 is sometimes 92 instead of 46.
(Solved)
Give a reason why the formula mass of NO2 is sometimes 92 instead of 46.
Date posted:
September 10, 2019
.
Answers (1)
-
Consider the chromatogram below.
(Solved)
Consider the chromatogram below.

A piece of chromatogram paper was spotted with colour inks obtained from pens labeled A to F. The diagram above shows the spots after the chromatograph was developed.
(a) Which two pens contained the same pigment?
(b) According to the chromatogram which pigments are present in the inks of the pen number F
(c) Describe how one could get a sample of yellow pigment
Date posted:
September 10, 2019
.
Answers (1)
-
Hydrazine gas, shown below, burns in oxygen to form nitrogen gas and steam.
(Solved)
Hydrazine gas, shown below, burns in oxygen to form nitrogen gas and steam.

(a) Write an equation for the reaction
(b) Using the bond energies given below, calculate the enthalpy change for the reaction in (a) above

Date posted:
September 10, 2019
.
Answers (1)
-
A form one class carried out an experiment to determine the active part of air. The diagram below shows the set-up of the experiment and...
(Solved)
A form one class carried out an experiment to determine the active part of air. The diagram below shows the set-up of the experiment and also the observation made.

(a) Identify substance M
(b) State two reasons for the suitability of substance M for this experiment
(c) Write the equation for the reaction of substance M and the active part of air
Date posted:
September 10, 2019
.
Answers (1)
-
Draw a dot and cross diagram to show bonding in sulphur (IV) oxide
(Solved)
Draw a dot and cross diagram to show bonding in sulphur (IV) oxide
Date posted:
September 10, 2019
.
Answers (1)
-
Give one use of charcoal in the sugar refinery industry.
(Solved)
Give one use of charcoal in the sugar refinery industry.
Date posted:
September 10, 2019
.
Answers (1)