- Complete the table below.(Solved)
Complete the table below.
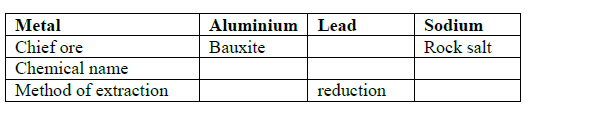
Date posted: September 12, 2019. Answers (1)
- Use the information in the table below to answer the questions that follow.(Solved)
Use the information in the table below to answer the questions that follow.

(a) Write the electron arrangement of:
(i) ion of S
(ii) atom of T
(b) Explain why the melting point of T is higher than that of U.
Date posted: September 12, 2019. Answers (1)
- You are given the following half equations:(Solved)
You are given the following half equations:
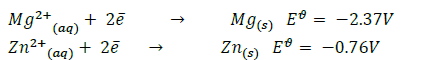
(i) Obtain an equation of the cell reaction.
(ii) Calculate the  value for the cell.
value for the cell.
(iii) Give the oxidizing species.
Date posted: September 12, 2019. Answers (1)
- The structures below represent two cleaning agents M and P.(Solved)
The structures below represent two cleaning agents M and P.
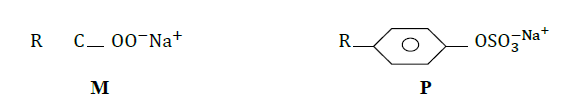
Which cleaning agent would be most suitable for use with water containing calcium sulphate. Give a reason.
Date posted: September 12, 2019. Answers (1)
- Use the nuclear equations below to answer the questions that follow.(Solved)
Use the nuclear equations below to answer the questions that follow.
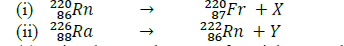
(a) Give the actual names of particles X and Y.
(b) Give the name of a radiation whose emission does not change the mass number or the atomic number of a radioisotope.
Date posted: September 12, 2019. Answers (1)
- Study the chart below and answer the questions that follow.(Solved)
Study the chart below and answer the questions that follow.
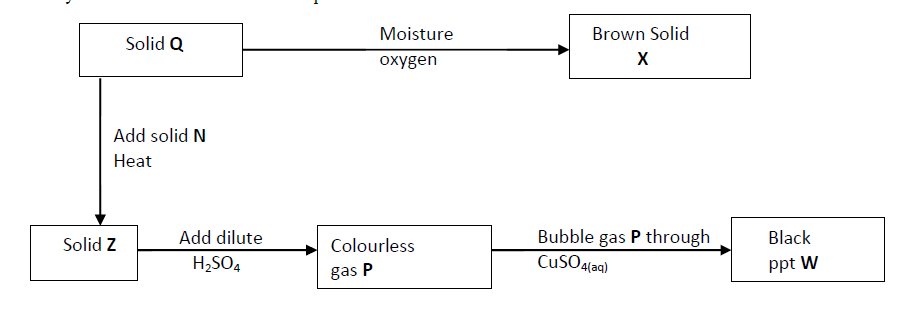
(a) Identity solid X.
(b) Write an ionic equation for the reaction between P and copper (II) sulphide solution.
(c) State the observation made when gas P is bubbled through iron (III) chloride solution.
Date posted: September 12, 2019. Answers (1)
- Perspex is a synthetic polymer of formula(Solved)
Perspex is a synthetic polymer of formula

(a) Write the structural formula of the monomer of Perspex.
(b) State the type of polymerization involved in the formation of perspex.
Date posted: September 12, 2019. Answers (1)
- When 8.8g of hydrocarbon Z was burnt in excess air, 14.4g of water and 11.95 dm3 of carbon (IV) oxide were obtained at s.t.p. Determine...(Solved)
When 8.8g of hydrocarbon Z was burnt in excess air, 14.4g of water and 11.95 dm3 of carbon (IV) oxide were obtained at s.t.p. Determine the empirical formula of Z.
Date posted: September 12, 2019. Answers (1)
- State the conditions under which copper reacts with sulphuric acid and give an equation for the reaction.(Solved)
State the conditions under which copper reacts with sulphuric acid and give an equation for the reaction.
Date posted: September 12, 2019. Answers (1)
- Use the thermochemical equations below to calculate the enthalpy of formation of ethane.
(Solved)
Use the thermochemical equations below to calculate the enthalpy of formation of ethane.
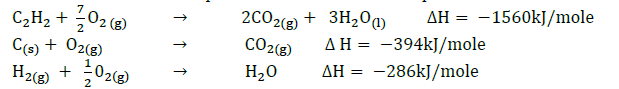
Date posted: September 12, 2019. Answers (1)
- Manganese sulphide reacts with acids according to the following equation.(Solved)
Manganese sulphide reacts with acids according to the following equation.
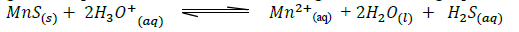
State, giving a reason what would happen to the equilibrium if;
(i) Water is added to the equilibrium mixture.
(ii) Hydrogen chloride is bubbled into the equilibrium mixture.
Date posted: September 12, 2019. Answers (1)
- Explain why flammable substances should always be kept away from flames in the laboratory.(Solved)
Explain why flammable substances should always be kept away from flames in the laboratory.
Date posted: September 12, 2019. Answers (1)
- When a sample of ethane was burnt, the heat provided raised the temperature of 500cm3 of water by 21.5k
(specific heat capacity of water = 4.2kJ/kg/k...(Solved)
When a sample of ethane was burnt, the heat provided raised the temperature of 500cm3 of water by 21.5k
(specific heat capacity of water = 4.2kJ/kg/k and density of water = 1g/cm3.
Calculate the:
(i) Heat change for the reaction.
(ii) Mass of ethane burnt (RFM of ethane = 30).
Date posted: September 12, 2019. Answers (1)
- Use the thermo chemical equations below to answer the questions that follow.(Solved)
Use the thermo chemical equations below to answer the questions that follow.

(i) Name two types of heat changes represent by 
(ii) Draw an energy level diagram for the reaction represented by equation 1.
(iii) Calculate the standard enthalpy of formation of ethane.
Date posted: September 12, 2019. Answers (1)
- Study the following part of periodic table chart and use it to answer the question that follow. The letters are of the actual symbols of...(Solved)
Study the following part of periodic table chart and use it to answer the question that follow. The letters are of the actual symbols of the elements.

(i) Which elements form ions with charge of -2. Explain.
(ii) Compare the ionic radius of C and E. Explain.
(iii) Write the formular of the compound formed between element G and H.
(iv) In terms of structure of bonding, explain why the oxides of D has a lower melting point than that of G.
(v) Write an equation to show the action of heat on nitrates of F and G.
Date posted: September 12, 2019. Answers (1)
- The reaction between Lead (II) Nitrate and concentrated sulphuric (VI) acid starts but stops immediately.Explain.(Solved)
The reaction between Lead (II) Nitrate and concentrated sulphuric (VI) acid starts but stops immediately.Explain.
Date posted: September 12, 2019. Answers (1)
- The table below gives the solubilities of potassium nitrate at different temperatures.(Solved)
The table below gives the solubilities of potassium nitrate at different temperatures.

(i) Plot a graph of the solubility of potassium nitrate (vertical axis) against temperature.
(ii) Using the graph:
I. Determine the solubility of potassium nitrate at 150C.
II. Determine the mass of potassium nitrate that remained undissolved given that 80g of potassium nitrate were added to 100cm3 of water and warmed to 400C. (2mks)
(iii) Determine the molar concentration of potassium nitrate at 1500C. (Assume there is no change in density of water at this
temperature) (K = 39.0; N = 14.0; O = 16.0)
Date posted: September 12, 2019. Answers (1)
- Draw the structural formula of Butyne(Solved)
Draw the structural formula of Butyne
Date posted: September 12, 2019. Answers (1)
- Use standard electrode potentials of elements A, B, C, D and F given below to answer the questions that follow. The letters do not represent...(Solved)
Use standard electrode potentials of elements A, B, C, D and F given below to answer the questions that follow. The letters do not represent the actual symbols of the elements.

(i) Which element is likely to be hydrogen? Give a reason for your answer.
(ii) What is the  value of the strongest reducing agent?
value of the strongest reducing agent?
(iii) In the space provided, draw a labeled diagram of the electrochemical cell that would be obtained when half-cells of element B and D are combined.
(iv) Calculate  the value of the electrochemical cell constructed in (iii) above.
the value of the electrochemical cell constructed in (iii) above.
Date posted: September 12, 2019. Answers (1)
- Study the flow chart below and answer the question that follow.(Solved)
Study the flow chart below and answer the question that follow.

Name the substances: C and D
Date posted: September 12, 2019. Answers (1)