-
The products formed by action of heat on nitrates of element X, Y and Z are shown below.
(Solved)
The products formed by action of heat on nitrates of element X, Y and Z are shown below.

a) Arrange the metals in order of increasing reactivity.
b) Which element forms a soluble carbonate?
c) Give an element that can be Y.
Date posted:
September 17, 2019
.
Answers (1)
-
a) Draw a diagram to show how an iron ring can be electroplated with pure silver.
b)Give two reasons why electroplating is necessary.
(Solved)
a) Draw a diagram to show how an iron ring can be electroplated with pure silver.
b)Give two reasons why electroplating is necessary.
Date posted:
September 17, 2019
.
Answers (1)
-
Consider the flow chart below; use it to answer the questions that follow.
(Solved)
Consider the flow chart below; use it to answer the questions that follow.

(i) State the most likely identity of solid F.
(ii) Write the chemical equation for the reaction between solid F and concentrated nitric (v) acid.
(iii) Name
Solution N
Solution H
(iv) Write the formula of solution N.
Date posted:
September 17, 2019
.
Answers (1)
-
Name the class to which the following cleansing agents belong.
(Solved)
a)Name the class to which the following cleansing agents belong.

b) Which cleaning agent between (i) or (ii) above is preferred for cleaning garments while using water from a dam containing dissolved calcium chloride? Explain
Date posted:
September 17, 2019
.
Answers (1)
-
Describe how a solid mixture of Zinc sulphate and lead (ii) Sulphate can be separated into Solid samples.
(Solved)
Describe how a solid mixture of Zinc sulphate and lead (ii) Sulphate can be separated into Solid samples.
Date posted:
September 17, 2019
.
Answers (1)
-
Other than cost and ability to conduct, give two other reasons why aluminum is used for making overhead electric cables while magnesium is not.
(Solved)
Other than cost and ability to conduct, give two other reasons why aluminum is used for making overhead electric cables while magnesium is not.
Date posted:
September 17, 2019
.
Answers (1)
-
A student set up the apparatus as shown in the diagram below to prepare and collect dry ammonia gas.
(Solved)
A student set up the apparatus as shown in the diagram below to prepare and collect dry ammonia gas.

(i) Identify one mistake in the set-up and give a reason.
(ii) Name a suitable drying agent for ammonia.
(iii) Write an operation for the reaction that occurred when a mixture of ammonium chloride and calcium hydroxide was heated.
(iv) Describe one chemical test for ammonia gas.
Date posted:
September 17, 2019
.
Answers (1)
-
Study the reaction scheme below and answer the questions that follow.
(Solved)
Study the reaction scheme below and answer the questions that follow.

a) Name; Gas M
Precipitate w
b) Write an equation for the formation of ;
The white solid
Colorless solution X
c) What name is given to the process that produce precipitate W
d) State two commercial uses of gas M
Date posted:
September 17, 2019
.
Answers (1)
-
Below is a simplified diagram of the Downs cell in which sodium metal is manufactured.
(Solved)
Below is a simplified diagram of the Downs cell in which sodium metal is manufactured.

(i) Identify electrolyte X and gas Y.
(ii)Give two properties of sodium that make it possible to collect as shown above.
(iii) Why is graphite preferred to steel as the anode?
(iv) Explain how sodium hydroxide pellets would be obtained from sodium amalgam?
Date posted:
September 17, 2019
.
Answers (1)
-
Compute the molar heat of combustion of butane, given that
(Solved)
Given that

Date posted:
September 17, 2019
.
Answers (1)
-
The figure below represents a section of the periodic table. Study it and answer the questions that follow. The letters do not represent the actual...
(Solved)
The figure below represents a section of the periodic table. Study it and answer the questions that follow. The letters do not represent the actual symbols of elements.

a) i).What chemical family does element J belong to?
ii. Compare the reactivity of element C and H. Explain.
b)
i) Write the chemical formula of the chloride of element D
ii) Name the type of structure of the chloride in b(i) above.
c). State and explain the difference in atomic radius and ionic radius of element F
d). Using dots (.) and crosses (x) show how bonding occurs when element E and F react.
Date posted:
September 17, 2019
.
Answers (1)
-
The flow chart below and answer the questions that follow.
(Solved)
The flow chart below and answer the questions that follow.

i). Identify the following;
Gas R
Solid M
Gas J
Liquid S
ii. Write a chemical equation for the formation of gas T
iii. Name catalyst K
iv) State two uses of liquid S
Date posted:
September 17, 2019
.
Answers (1)
-
60cm3 of oxygen gas diffused through a porous partition in 50 seconds. How long will it take 120cm3 of sulphur (IV) oxide gas to diffuse...
(Solved)
60cm3 of oxygen gas diffused through a porous partition in 50 seconds. How long will it take 120cm3 of sulphur (IV) oxide gas to diffuse through the same partition under the same conditions.
Date posted:
September 17, 2019
.
Answers (1)
-
In an experiment magnesium ribbon was reacted with dilute sulphuric (VI) acid and the volume of hydrogen gas produced with time noted. The graph below...
(Solved)
In an experiment magnesium ribbon was reacted with dilute sulphuric (VI) acid and the volume of hydrogen gas produced with time noted. The graph below shows the volume of gas produced with time

Explain the following observations.
(i) The curve of the graph is steepest at the beginning.
(ii) The curve of the graph completely flattens at region AB.
(iii) On the same axis plot the curve that would be obtained if the acid used was ethanoic acid. Label it ethanoic acid
Date posted:
September 17, 2019
.
Answers (1)
-
The table below gives the solubility of potassium bromide and potassium sulphate at 00C and 800C.
(Solved)
The table below gives the solubility of potassium bromide and potassium sulphate at 00C and 800C.

When an aqueous mixture containing 60g of potassium bromide and 7g of potassium sulphate in 100g of water was cooled from 800C to 00C, some crystals were formed.
(a) Identify the crystals.
(b) Determine the mass of crystals formed.
(c) Name the method used to obtain the crystals.
Date posted:
September 17, 2019
.
Answers (1)
-
Describe how solid Aluminium chloride can be separated from a solid mixture of sodium chloride and ammonium chloride.
(Solved)
Describe how solid Aluminium chloride can be separated from a solid mixture of sodium chloride and ammonium chloride.
Date posted:
September 17, 2019
.
Answers (1)
-
The relative atomic mass of an element is 10.28.
(Solved)
The relative atomic mass of an element is 10.28, it has two isotopes 
Calculate the relative percentage abundance of each isotope.
Date posted:
September 17, 2019
.
Answers (1)
-
The atomic number of Q and R are 9 and 17 respectively. Compare the electron affinity of Q and . Explain.
(Solved)
The atomic number of Q and R are 9 and 17 respectively. Compare the electron affinity of Q and . Explain.
Date posted:
September 17, 2019
.
Answers (1)
-
In order to determine the molar heat of neutralization of 1M potassium hydroxide, 200cm³ of 1M hydrochloric acid both at the same temperature were mixed...
(Solved)
In order to determine the molar heat of neutralization of 1M potassium hydroxide, 200cm³ of 1M hydrochloric acid both at the same temperature were mixed and stirred continuously with a thermometer. The temperature of the resulting solution was recorded after every 30 seconds until the highest temperature of the solution was attained.
(a) (i) Why was it necessary to stir the mixture of the two solutions?
(ii) Write an ionic equation for the reaction.
(b) The initial temperature for both solution was 24.5°C and the highest temperature attained by the mixture was 30.9°C. Calculate the
(i) heat change for the reaction.
(Specific heat capacity of the solution is 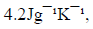 Density of the solution is
Density of the solution is 
The volume of KOH used was 200cm³.
(ii) molar heat of the neutralisation.
(c) If ammonium hydroxide was used instead of potassium hydroxide the heat of neutralization would be different from the one obtained in b(ii) above.
Explain the difference.
(d) Draw an energy level diagram for the reaction between potassium hydroxide and hydrochloric acid.
Date posted:
September 16, 2019
.
Answers (1)
-
Explain why lead carbonate is not reacted with dil. H2SO4 in preparation of carbon (IV) oxide in the laboratory.
(Solved)
Explain why lead carbonate is not reacted with dil. H2SO4 in preparation of carbon (IV) oxide in the laboratory.
Date posted:
September 16, 2019
.
Answers (1)