2M Sulphuric (VI) Acid has a higher ammeter reading than 2 M ethanoic acid because it is a stronger acid and it ionizes in water fully than ethanoic acid ionizes partially.
maurice.mutuku answered the question on September 19, 2019 at 06:29
- Study the energy level diagram for the reaction shown below and use it to answer the questions that follow.(Solved)
Study the energy level diagram for the reaction shown below and use it to answer the questions that follow.
2SO2(g) + O2(g)----->2SO3(g)
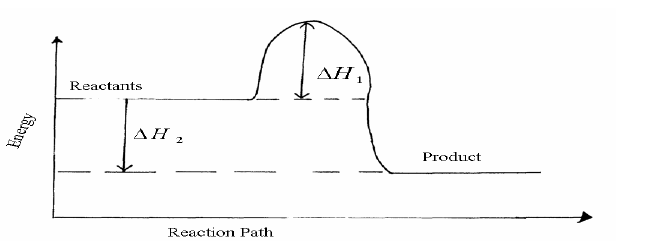
(i) State and explain two ways of increasing the yield of SO3 per unit time from the diagram.
(ii) What do the following represent?
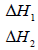
Date posted: September 19, 2019. Answers (1)
- When Iron (III) chloride is exposed to the atmosphere, it forms a solution.
(a) Name the process that takes place.
(b) State one use of the...(Solved)
When Iron (III) chloride is exposed to the atmosphere, it forms a solution.
(a) Name the process that takes place.
(b) State one use of the process named in (a) above.
Date posted: September 19, 2019. Answers (1)
- The following diagram was used to study a property of hydrogen gas. Study it and answer the questions that follow.(Solved)
The following diagram was used to study a property of hydrogen gas. Study it and answer the questions that follow.

a) Name the missing condition in the above set up.
b) Explain why the combustion tube is clamped in a slanting position.
c) Before lighting the gas at the end of delivery tube, hydrogen must be let to pass through until all the air is driven out. Explain.
d) State three observations that occur in the combustion tube.
e) Why was hydrogen gas burnt at point Z.
f) Why should the supply of hydrogen gas be continued while the apparatus cool.
g) What would be observed if the experiment was repeated using lead (II) oxide.
h) Other than the property investigated above, name two other chemical properties of hydrogen gas.
Date posted: September 19, 2019. Answers (1)
- Find the mass of 5.2 x 1025 atoms of sodium. (Na = 23.0, L = 6.023 x 103)(Solved)
Find the mass of 5.2 x 1025 atoms of sodium. (Na = 23.0, L = 6.023 x 103)
Date posted: September 19, 2019. Answers (1)
- 0.239g of copper (II) oxide was placed in a conical flask. Calculate the volume of 0.1 M solution of hydrochloric acid that would completely react...(Solved)
0.239g of copper (II) oxide was placed in a conical flask. Calculate the volume of 0.1 M solution of hydrochloric acid that would completely react with copper (II) oxide in the conical flask.
(0= 16.0, Cu = 63.5, H= 1.0, Cl = 35.5)
Date posted: September 19, 2019. Answers (1)
- Calculate the mass of sodium carbonate contained in 200cm3 of 0.02M sodium carbonate solution.(Solved)
Calculate the mass of sodium carbonate contained in 200cm3 of 0.02M sodium carbonate solution.
Date posted: September 19, 2019. Answers (1)
- A sample of 10cm3 of hydrogen sulphide was burned in 40cm3 of oxygen. Calculate the volume and composition of residual gas (assume all volumes are...(Solved)
A sample of 10cm3 of hydrogen sulphide was burned in 40cm3 of oxygen. Calculate the volume and composition of residual gas (assume all volumes are measured at s.t.p)
Date posted: September 19, 2019. Answers (1)
- What is Molar gas volume?(Solved)
What is Molar gas volume?
Date posted: September 19, 2019. Answers (1)
- The diagram below represents a set up that was used for electrolysis of aqueous copper (II) nitrate.(Solved)
The diagram below represents a set up that was used for electrolysis of aqueous copper (II) nitrate.

A gas that relights a glowing splint was produced at electrode A.
i) Which electrode is the cathode ? Explain.
ii) State another suitable method for collecting gas X.
iii) Write ionic equations to show reactions that take place at the :
Anode
Cathode
iv) Explain how the identity of the product at the cathode of this electrolysis can be confirmed.
v) Calculate the mass of copper deposited if a constant current of 5 A was passed for 3 hours. (Cu = 63.5, IF = 965000)
Date posted: September 18, 2019. Answers (1)
- Study the table below and answer the questions that follow. The letters do not represents the actual symbols of the elements(Solved)
Study the table below and answer the questions that follow. The letters do not represents the actual symbols of the elements.

Select elements found in:
(a) (i) The same group
(ii) Period three
(iii) What is the family name given to the group to which elements identified in a(i) above belongs.
(b) How does the atomic radius of element B and A compare. Explain.
(c) State two industrial use of element B.
(d) With reason, compare the reactivity of C and D.
(e) Write the formula of compound formed when A and D react.
(f) What type of bond is formed when element E react with oxygen. Give a reason for your answer.
Date posted: September 18, 2019. Answers (1)
- Excess concentrated sulphuric VI acid was mixed with pieces of dry wood as shown.(Solved)
Excess concentrated sulphuric VI acid was mixed with pieces of dry wood as shown.

a) State the observation made in the tube.
b) When the reaction was complete, the mixture was heated gently, then strongly and set up adjusted as shown below.

State and explain the observation made on acidified potassium chromate VI solution.
Date posted: September 18, 2019. Answers (1)
- The cell convention for and electrochemical cell is shown below.(Solved)
The cell convention for and electrochemical cell is shown below.

(a) Name two substances that can be used as electrolytes in the above cell.
(b) Which of the electrodes is the negative in the cell above ? Explain.
Date posted: September 18, 2019. Answers (1)
- The table below shows properties of some chlorides. Study it and answer the questions that follow.(Solved)
The table below shows properties of some chlorides. Study it and answer the questions that follow.

a) Explain the high melting and boiling points of sodium chloride.
b) Write an equation for the reaction between PC15 and water.
c) Draw the dot (•) and cross (x) diagram to show bonding in NaCl.
Date posted: September 18, 2019. Answers (1)
- The labels of two reagent bottles contained the following safety symbols.(Solved)
The labels of two reagent bottles contained the following safety symbols.

a) What do the symbols mean ? Explain.
b) Which of the reagent is more harmful ?
Date posted: September 18, 2019. Answers (1)
- What volume of oxygen gas at r.t.p will be liberated at the anode when a current of 3 amperes is passed through magnesium sulphate solution...(Solved)
What volume of oxygen gas at r.t.p will be liberated at the anode when a current of 3 amperes is passed through magnesium sulphate solution for 45 minutes and 30 seconds. (Molar gas volume at r.t.p = 24000cm3, Faraday constant = 96500
Coulombs)
Date posted: September 18, 2019. Answers (1)
- When bismuth III Chloride is added to water, a reaction occurs and a white precipitate forms as shown below.
BiCI3(aq) + H20(l) BiOCl(s) + 2HCl(aq)
What would...(Solved)
When bismuth III Chloride is added to water, a reaction occurs and a white precipitate forms as shown below.
BiCI3(aq) + H20(l) BiOCl(s) + 2HCl(aq)
What would be the effect on the amount of the precipitate formed if sodium hydroxide solution is added to the equilibrium mixture ? Explain your answer.
Date posted: September 18, 2019. Answers (1)
- When 10g of a mixture of potassium chloride and anhydrous sodium sulphate is dissolved in water and excess barium chloride solution added. 6.9g of Barium...(Solved)
When 10g of a mixture of potassium chloride and anhydrous sodium sulphate is dissolved in water and excess barium chloride solution added. 6.9g of Barium sulphate is precipitated. Calculate the composition of the mixture. (K .= 39. Cl =35.5, Na=23, O =16, Ba=137)
Date posted: September 18, 2019. Answers (1)
- Explain why iron nails rust faster in sodium chloride solution than in tap water.(Solved)
Explain why iron nails rust faster in sodium chloride solution than in tap water.
Date posted: September 18, 2019. Answers (1)
- Given the following reagents; solid sodium carbonate, solid lead (II) nitrate, water. Describe how a sample of lead (II) carbonate can be prepared in the...(Solved)
Given the following reagents; solid sodium carbonate, solid lead (II) nitrate, water. Describe how a sample of lead (II) carbonate can be prepared in the laboratory.
Date posted: September 18, 2019. Answers (1)
- Radioactive polonium - 216 decays as shown below.(Solved)
Radioactive polonium - 216 decays as shown below.

Date posted: September 18, 2019. Answers (1)