- Methane gas reacts with chlorine gas as shown in the equation below.(Solved)
Methane gas reacts with chlorine gas as shown in the equation below.

Use the bond energies in the table below to calculate the enthalpy for the above reaction.

Date posted: September 19, 2019. Answers (1)
- The set- up below was used to investigate the rate of diffusion of different gases.(Solved)
The set- up below was used to investigate the rate of diffusion of different gases.
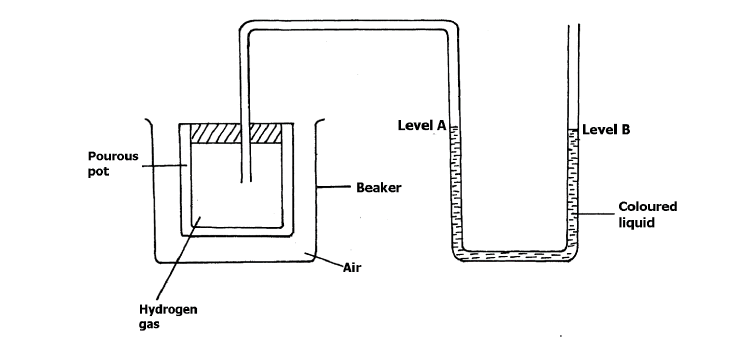
a) Explain why a coloured liquid is used in this experiment.
b) State and explain the observation made after 20 minutes.
Date posted: September 19, 2019. Answers (1)
- The table below gives information about some reactions of metals A,B, C and D and their rates.(Solved)
The table below gives information about some reactions of metals A,B, C and D and their rates.
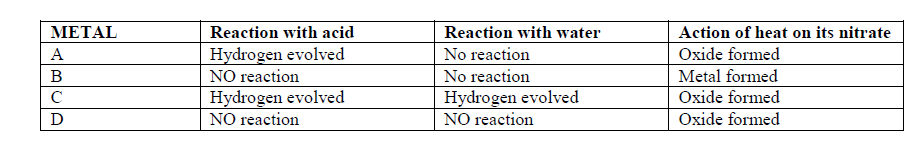
Arrange the metals in order of decreasing activity.
Date posted: September 19, 2019. Answers (1)
- The diagram below shows the set-up that can be used to prepare and collect oxygen gas. Study it and answer the questions that follow.(Solved)
The diagram below shows the set-up that can be used to prepare and collect oxygen gas. Study it and answer the questions that follow.
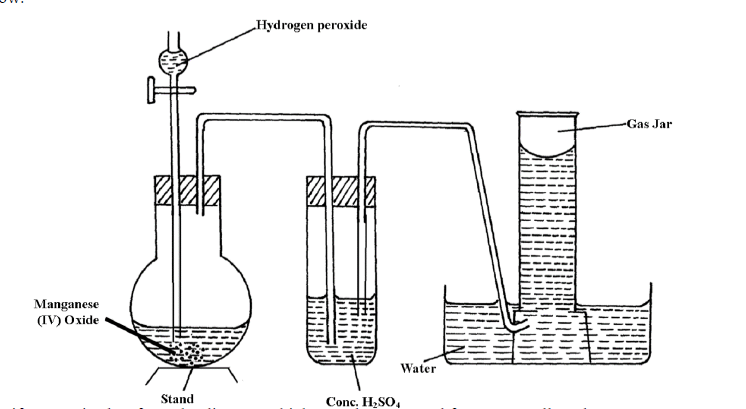
Identify two mistakes from the diagram which must be corrected for one to collect dry oxygen gas.
Date posted: September 19, 2019. Answers (1)
- State the conditions under which ammonia gives the following products when heated
(i) Nitrogen and hydrogen.
(ii) Nitrogen and water.
(iii) Nitrogen (II) oxide and water.(Solved)
State the conditions under which ammonia gives the following products when heated
(i) Nitrogen and hydrogen.
(ii) Nitrogen and water.
(iii) Nitrogen (II) oxide and water.
Date posted: September 19, 2019. Answers (1)
- The solubility of salt T at 80oC is 40g / 100g of water. What mass of T will saturate 65g of water at 80oC?(Solved)
The solubility of salt T at 80oC is 40g / 100g of water. What mass of T will saturate 65g of water at 80oC?
Date posted: September 19, 2019. Answers (1)
- Using electrons in the outermost energy level, draw a dot (.) and cross (X) diagram for the ion and compound given. (P=15, H=1, B=5,...(Solved)
Using electrons in the outermost energy level, draw a dot (.) and cross (X) diagram for the ion of 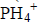 and compound B2O3. (P=15, H=1, B=5, O= 16)
and compound B2O3. (P=15, H=1, B=5, O= 16)
Date posted: September 19, 2019. Answers (1)
- The table below shows ammeter reading recorded when 2M Sulphuric (VI) acid and 2M ethanoic acid were tested separately.(Solved)
The table below shows ammeter reading recorded when 2M Sulphuric (VI) acid and 2M ethanoic acid were tested separately.
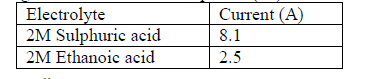
Explain the difference in the ammeter readings.
Date posted: September 19, 2019. Answers (1)
- Study the energy level diagram for the reaction shown below and use it to answer the questions that follow.(Solved)
Study the energy level diagram for the reaction shown below and use it to answer the questions that follow.
2SO2(g) + O2(g)----->2SO3(g)
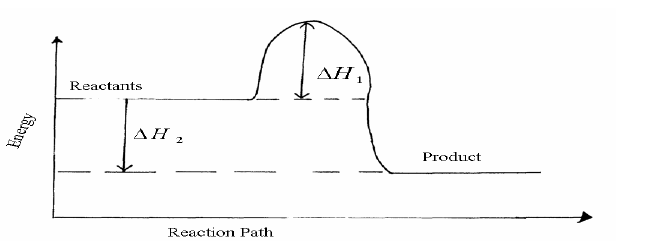
(i) State and explain two ways of increasing the yield of SO3 per unit time from the diagram.
(ii) What do the following represent?
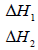
Date posted: September 19, 2019. Answers (1)
- When Iron (III) chloride is exposed to the atmosphere, it forms a solution.
(a) Name the process that takes place.
(b) State one use of the...(Solved)
When Iron (III) chloride is exposed to the atmosphere, it forms a solution.
(a) Name the process that takes place.
(b) State one use of the process named in (a) above.
Date posted: September 19, 2019. Answers (1)
- The following diagram was used to study a property of hydrogen gas. Study it and answer the questions that follow.(Solved)
The following diagram was used to study a property of hydrogen gas. Study it and answer the questions that follow.

a) Name the missing condition in the above set up.
b) Explain why the combustion tube is clamped in a slanting position.
c) Before lighting the gas at the end of delivery tube, hydrogen must be let to pass through until all the air is driven out. Explain.
d) State three observations that occur in the combustion tube.
e) Why was hydrogen gas burnt at point Z.
f) Why should the supply of hydrogen gas be continued while the apparatus cool.
g) What would be observed if the experiment was repeated using lead (II) oxide.
h) Other than the property investigated above, name two other chemical properties of hydrogen gas.
Date posted: September 19, 2019. Answers (1)
- Find the mass of 5.2 x 1025 atoms of sodium. (Na = 23.0, L = 6.023 x 103)(Solved)
Find the mass of 5.2 x 1025 atoms of sodium. (Na = 23.0, L = 6.023 x 103)
Date posted: September 19, 2019. Answers (1)
- 0.239g of copper (II) oxide was placed in a conical flask. Calculate the volume of 0.1 M solution of hydrochloric acid that would completely react...(Solved)
0.239g of copper (II) oxide was placed in a conical flask. Calculate the volume of 0.1 M solution of hydrochloric acid that would completely react with copper (II) oxide in the conical flask.
(0= 16.0, Cu = 63.5, H= 1.0, Cl = 35.5)
Date posted: September 19, 2019. Answers (1)
- Calculate the mass of sodium carbonate contained in 200cm3 of 0.02M sodium carbonate solution.(Solved)
Calculate the mass of sodium carbonate contained in 200cm3 of 0.02M sodium carbonate solution.
Date posted: September 19, 2019. Answers (1)
- A sample of 10cm3 of hydrogen sulphide was burned in 40cm3 of oxygen. Calculate the volume and composition of residual gas (assume all volumes are...(Solved)
A sample of 10cm3 of hydrogen sulphide was burned in 40cm3 of oxygen. Calculate the volume and composition of residual gas (assume all volumes are measured at s.t.p)
Date posted: September 19, 2019. Answers (1)
- What is Molar gas volume?(Solved)
What is Molar gas volume?
Date posted: September 19, 2019. Answers (1)
- The diagram below represents a set up that was used for electrolysis of aqueous copper (II) nitrate.(Solved)
The diagram below represents a set up that was used for electrolysis of aqueous copper (II) nitrate.

A gas that relights a glowing splint was produced at electrode A.
i) Which electrode is the cathode ? Explain.
ii) State another suitable method for collecting gas X.
iii) Write ionic equations to show reactions that take place at the :
Anode
Cathode
iv) Explain how the identity of the product at the cathode of this electrolysis can be confirmed.
v) Calculate the mass of copper deposited if a constant current of 5 A was passed for 3 hours. (Cu = 63.5, IF = 965000)
Date posted: September 18, 2019. Answers (1)
- Study the table below and answer the questions that follow. The letters do not represents the actual symbols of the elements(Solved)
Study the table below and answer the questions that follow. The letters do not represents the actual symbols of the elements.

Select elements found in:
(a) (i) The same group
(ii) Period three
(iii) What is the family name given to the group to which elements identified in a(i) above belongs.
(b) How does the atomic radius of element B and A compare. Explain.
(c) State two industrial use of element B.
(d) With reason, compare the reactivity of C and D.
(e) Write the formula of compound formed when A and D react.
(f) What type of bond is formed when element E react with oxygen. Give a reason for your answer.
Date posted: September 18, 2019. Answers (1)
- Excess concentrated sulphuric VI acid was mixed with pieces of dry wood as shown.(Solved)
Excess concentrated sulphuric VI acid was mixed with pieces of dry wood as shown.

a) State the observation made in the tube.
b) When the reaction was complete, the mixture was heated gently, then strongly and set up adjusted as shown below.

State and explain the observation made on acidified potassium chromate VI solution.
Date posted: September 18, 2019. Answers (1)
- The cell convention for and electrochemical cell is shown below.(Solved)
The cell convention for and electrochemical cell is shown below.

(a) Name two substances that can be used as electrolytes in the above cell.
(b) Which of the electrodes is the negative in the cell above ? Explain.
Date posted: September 18, 2019. Answers (1)