- When solid Zinc carbonate was added to a solution of hydrogen chloride in methylbenzene, there was no observable change.On addition of some water to the...(Solved)
When solid Zinc carbonate was added to a solution of hydrogen chloride in methylbenzene, there was no observable change.On addition of some water to the mixture there was effervescence. Explain these observations.
Date posted: September 19, 2019. Answers (1)
- Graphite is an allotrope of carbon. Distinguish between allotropes and isotopes.(Solved)
Graphite is an allotrope of carbon. Distinguish between allotropes and isotopes.
Date posted: September 19, 2019. Answers (1)
- Graphite is a non- metal most commonly used as an electrode. State two properties that makes it suitable for use as an electrode.(Solved)
Graphite is a non- metal most commonly used as an electrode. State two properties that makes it suitable for use as an electrode.
Date posted: September 19, 2019. Answers (1)
- The equations show some reactions. Use the equations to answer the following questions.(Solved)
The equations show some reactions. Use the equations to answer the following questions.

(a) Name the type of reaction in step I and II.
(b) Explain why ethene burns with a more smoky flame than ethane.
Date posted: September 19, 2019. Answers (1)
- The diagram below shows the heating curve of a pure substance. Study it and answer the questions that follow.(Solved)
The diagram below shows the heating curve of a pure substance. Study it and answer the questions that follow.

(a) What are the physical states of the substances at points W and Y.
(b) Explain why the temperature remains constant between points B and C.
Date posted: September 19, 2019. Answers (1)
- Potassium manganate (VII) reacts with chloride salts to produce chlorine. Both chlorine and potassium manganate (VII) are strong oxidizing agents. Which one of the two...(Solved)
Potassium manganate (VII) reacts with chloride salts to produce chlorine. Both chlorine and potassium manganate (VII) are strong oxidizing agents. Which one of the two is the stronger oxidizing agent? Explain your answer
Date posted: September 19, 2019. Answers (1)
- When a grey powder P, which has no action on cold water is placed into a salt solution of Q, a brown solid R is...(Solved)
When a grey powder P, which has no action on cold water is placed into a salt solution of Q, a brown solid R is deposited.
The blue solution of Q, fades giving way to a green solution.
a) Name the type of reaction that takes place.
b) Identify solids P and R
c) Write an equation for the reaction leading to formation of the brown solid.
Date posted: September 19, 2019. Answers (1)
- A student dissolved some ammonium nitrate salt in water in a glass beaker. The solution formed felt very cold.(Solved)
A student dissolved some ammonium nitrate salt in water in a glass beaker. The solution formed felt very cold.
a) Explain why the temperature of the resultant solution dropped
b) Represent the above information on an energy level diagram
c) What general name is given to such reactions
Date posted: September 19, 2019. Answers (1)
- Methane gas reacts with chlorine gas as shown in the equation below.(Solved)
Methane gas reacts with chlorine gas as shown in the equation below.

Use the bond energies in the table below to calculate the enthalpy for the above reaction.

Date posted: September 19, 2019. Answers (1)
- The set- up below was used to investigate the rate of diffusion of different gases.(Solved)
The set- up below was used to investigate the rate of diffusion of different gases.
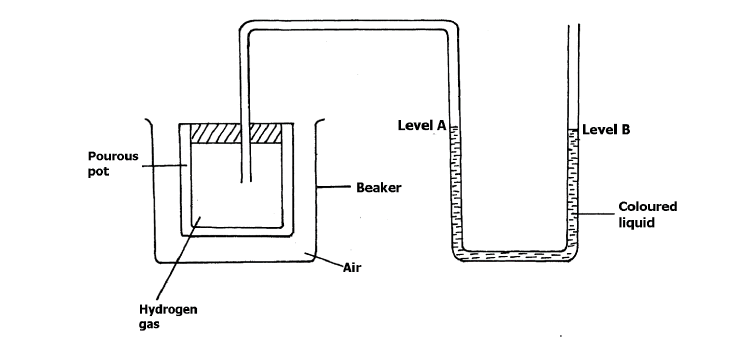
a) Explain why a coloured liquid is used in this experiment.
b) State and explain the observation made after 20 minutes.
Date posted: September 19, 2019. Answers (1)
- The table below gives information about some reactions of metals A,B, C and D and their rates.(Solved)
The table below gives information about some reactions of metals A,B, C and D and their rates.
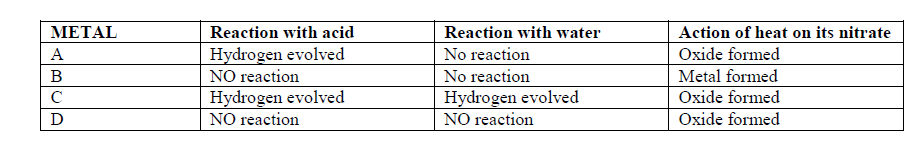
Arrange the metals in order of decreasing activity.
Date posted: September 19, 2019. Answers (1)
- The diagram below shows the set-up that can be used to prepare and collect oxygen gas. Study it and answer the questions that follow.(Solved)
The diagram below shows the set-up that can be used to prepare and collect oxygen gas. Study it and answer the questions that follow.
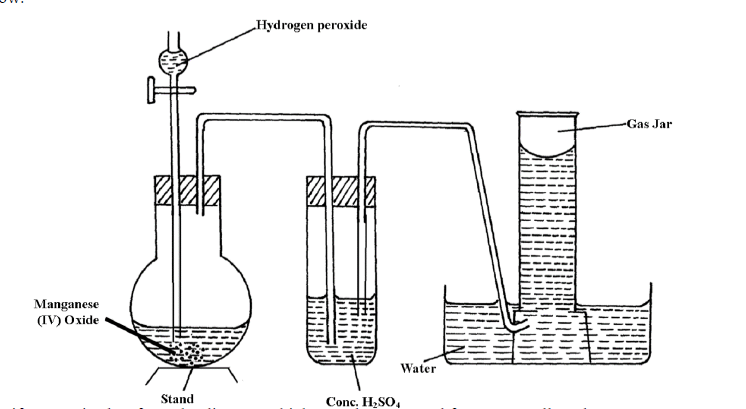
Identify two mistakes from the diagram which must be corrected for one to collect dry oxygen gas.
Date posted: September 19, 2019. Answers (1)
- State the conditions under which ammonia gives the following products when heated
(i) Nitrogen and hydrogen.
(ii) Nitrogen and water.
(iii) Nitrogen (II) oxide and water.(Solved)
State the conditions under which ammonia gives the following products when heated
(i) Nitrogen and hydrogen.
(ii) Nitrogen and water.
(iii) Nitrogen (II) oxide and water.
Date posted: September 19, 2019. Answers (1)
- The solubility of salt T at 80oC is 40g / 100g of water. What mass of T will saturate 65g of water at 80oC?(Solved)
The solubility of salt T at 80oC is 40g / 100g of water. What mass of T will saturate 65g of water at 80oC?
Date posted: September 19, 2019. Answers (1)
- Using electrons in the outermost energy level, draw a dot (.) and cross (X) diagram for the ion and compound given. (P=15, H=1, B=5,...(Solved)
Using electrons in the outermost energy level, draw a dot (.) and cross (X) diagram for the ion of 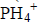 and compound B2O3. (P=15, H=1, B=5, O= 16)
and compound B2O3. (P=15, H=1, B=5, O= 16)
Date posted: September 19, 2019. Answers (1)
- The table below shows ammeter reading recorded when 2M Sulphuric (VI) acid and 2M ethanoic acid were tested separately.(Solved)
The table below shows ammeter reading recorded when 2M Sulphuric (VI) acid and 2M ethanoic acid were tested separately.
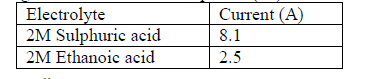
Explain the difference in the ammeter readings.
Date posted: September 19, 2019. Answers (1)
- Study the energy level diagram for the reaction shown below and use it to answer the questions that follow.(Solved)
Study the energy level diagram for the reaction shown below and use it to answer the questions that follow.
2SO2(g) + O2(g)----->2SO3(g)
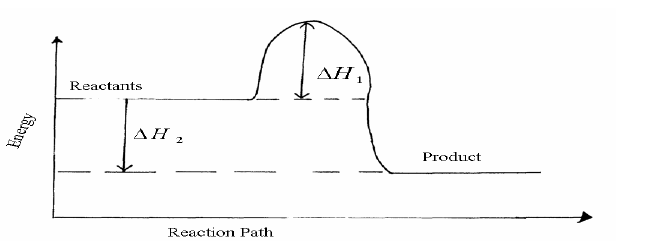
(i) State and explain two ways of increasing the yield of SO3 per unit time from the diagram.
(ii) What do the following represent?
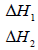
Date posted: September 19, 2019. Answers (1)
- When Iron (III) chloride is exposed to the atmosphere, it forms a solution.
(a) Name the process that takes place.
(b) State one use of the...(Solved)
When Iron (III) chloride is exposed to the atmosphere, it forms a solution.
(a) Name the process that takes place.
(b) State one use of the process named in (a) above.
Date posted: September 19, 2019. Answers (1)
- The following diagram was used to study a property of hydrogen gas. Study it and answer the questions that follow.(Solved)
The following diagram was used to study a property of hydrogen gas. Study it and answer the questions that follow.

a) Name the missing condition in the above set up.
b) Explain why the combustion tube is clamped in a slanting position.
c) Before lighting the gas at the end of delivery tube, hydrogen must be let to pass through until all the air is driven out. Explain.
d) State three observations that occur in the combustion tube.
e) Why was hydrogen gas burnt at point Z.
f) Why should the supply of hydrogen gas be continued while the apparatus cool.
g) What would be observed if the experiment was repeated using lead (II) oxide.
h) Other than the property investigated above, name two other chemical properties of hydrogen gas.
Date posted: September 19, 2019. Answers (1)
- Find the mass of 5.2 x 1025 atoms of sodium. (Na = 23.0, L = 6.023 x 103)(Solved)
Find the mass of 5.2 x 1025 atoms of sodium. (Na = 23.0, L = 6.023 x 103)
Date posted: September 19, 2019. Answers (1)