- Study the diagrams below.(Solved)
Study the diagrams below.

(a) State the observations made at I and II
(b) Write the equation to show the reactions at II if dry sulphur (IV) oxide was used in place of dry chlorine.
Date posted: September 20, 2019. Answers (1)
- Study the flow chart below and answer the questions that follow(Solved)
Study the flow chart below and answer the questions that follow

Process Y
i. Write the formula of Alcohol X, Compound Z and name process Y.
ii. Propane and Chlorine react as shown below :

a) Name the type of reaction that takes place.
b) State the condition under which this reaction takes place.
Date posted: September 20, 2019. Answers (1)
- Study the table below and answer the questions that follow(Solved)
Study the table below and answer the questions that follow

i) What name is given to a series of organic compounds like the ones in the table?
ii) To what class of organic compounds does the above hydrocarbon belongs?
iii) Select one hydrocarbon that would be a gas at room temperature (298K) given a reason for your answer.
iv) Give the formula of the seventh member of the above series.
v) What is the relationship between the boiling point and the relative molecular masses of the hydrocarbons in the table above?
Explain your answer.
Date posted: September 20, 2019. Answers (1)
- Study the scheme below and answer questions that follow.(Solved)
Study the scheme below and answer questions that follow.

i) Name
Gas K
Gas M
ii) State observation made in
Step I
Step II
iii) State the conditions necessary for step II to occur.
iv) Write an equation to show how pollution effect of sulphur (IV) oxide is controlled in contact process.
v) Explain the role of sulphur in vulcanization of rubber.
Date posted: September 20, 2019. Answers (1)
- Study the structure below and answer questions that follow.(Solved)
Study the structure below and answer questions that follow:

a) What observation is made when the molecule above is heated to a temperature of 1130 C
b) Write an equation for the reaction of atom of the above structure with hydrogen.
Date posted: September 20, 2019. Answers (1)
- Equal masses of magnesium ribbon were reacted separately with equal volumes of 1M hydrochloric acid and 1M methanoic acid. The results were plotted on a...(Solved)
Equal masses of magnesium ribbon were reacted separately with equal volumes of 1M hydrochloric acid and 1M methanoic acid. The results were plotted on a graph as shown below. Two curves X and Y were obtained.

a) Explain which curves represents:
i) 1M hydrochloric acid.
ii) 1M Methanoic acid.
b) State the significance of point Z.
c) Write ionic equation for the reaction between magnesium and dilute hydrochloric acid.
d) Calculate the maximum mass of the gas that would be liberated if 1.2g of magnesium reacted with excess hydrochloric acid.
(Mg = 24, H =1).
e) Calculate the volume of the gas produced in (e) above at r.t.p (molar gas volume at r.t.p) = 24dm3.
Date posted: September 20, 2019. Answers (1)
- The arrangements below shows a set-up to investigate the effect of an electric current on molten lead (II) iodide.(Solved)
The arrangements below shows a set-up to investigate the effect of an electric current on molten lead (II) iodide.

a) Identify two mistakes in the set-up.
b) State three observations made after correcting the mistakes.
c) What particles are responsible for electrical conductivity?
d) Write the equations for the reactions taking place at the electrodes.
e) Indicate on the diagram direction of flow of electric current.
Date posted: September 20, 2019. Answers (1)
- Study the information in the table below and answer the questions that follow, letters do not represent actual symbols of the element.(Solved)
Study the information in the table below and answer the questions that follow, letters do not represent actual symbols of the element.

a) Write the electronic configuration of an ion of elements T and U.
b) Why do the elements represented by R and S have two values of melting point.
c) Select an element:
i) Which is the most electronegative.
ii) That belongs to period 4, explain.
d) Explain why:
i) Ionic radius of R is bigger than its atomic radius.
ii) The atomic radius of L is bigger than that of R yet they are in the same period.
e) Using dots (.) and cross (x) to represent outermost electron show bonding in the compound formed between L and M.
f) Write an equation for the reaction that occurs between U and water.
g) Describe how a solid mixture of the sulphate of element N and lead (II) sulphate can be separated into solid sample of dry lead (II) sulphate.
Date posted: September 20, 2019. Answers (1)
- Study the set-up of apparatus below and answer the questions that follow.(Solved)
Study the set-up of apparatus below and answer the questions that follow.

a) State and explain the observation that would be made in tube L as the experiment progresses in the first few minutes.
Observation.
Explanation.
b) How would the observations in the tube L change if the experiment is carried out for a long time. Explain using a chemical equation.
Observation.
Equation.
c) State three observations made when liquid S is reacted with sodium metal.
d) State the use of the suction pump in this experiment.
Date posted: September 19, 2019. Answers (1)
- Study the chart below and answer the questions that follow.(Solved)
Study the chart below and answer the questions that follow.

(a) Name:
(i) Cations present in mixture X.
(ii) Anions present in the solution.
(b) Write an equation to show how the white precipitate in step III is formed.
Date posted: September 19, 2019. Answers (1)
- When solid Zinc carbonate was added to a solution of hydrogen chloride in methylbenzene, there was no observable change.On addition of some water to the...(Solved)
When solid Zinc carbonate was added to a solution of hydrogen chloride in methylbenzene, there was no observable change.On addition of some water to the mixture there was effervescence. Explain these observations.
Date posted: September 19, 2019. Answers (1)
- Graphite is an allotrope of carbon. Distinguish between allotropes and isotopes.(Solved)
Graphite is an allotrope of carbon. Distinguish between allotropes and isotopes.
Date posted: September 19, 2019. Answers (1)
- Graphite is a non- metal most commonly used as an electrode. State two properties that makes it suitable for use as an electrode.(Solved)
Graphite is a non- metal most commonly used as an electrode. State two properties that makes it suitable for use as an electrode.
Date posted: September 19, 2019. Answers (1)
- The equations show some reactions. Use the equations to answer the following questions.(Solved)
The equations show some reactions. Use the equations to answer the following questions.

(a) Name the type of reaction in step I and II.
(b) Explain why ethene burns with a more smoky flame than ethane.
Date posted: September 19, 2019. Answers (1)
- The diagram below shows the heating curve of a pure substance. Study it and answer the questions that follow.(Solved)
The diagram below shows the heating curve of a pure substance. Study it and answer the questions that follow.

(a) What are the physical states of the substances at points W and Y.
(b) Explain why the temperature remains constant between points B and C.
Date posted: September 19, 2019. Answers (1)
- Potassium manganate (VII) reacts with chloride salts to produce chlorine. Both chlorine and potassium manganate (VII) are strong oxidizing agents. Which one of the two...(Solved)
Potassium manganate (VII) reacts with chloride salts to produce chlorine. Both chlorine and potassium manganate (VII) are strong oxidizing agents. Which one of the two is the stronger oxidizing agent? Explain your answer
Date posted: September 19, 2019. Answers (1)
- When a grey powder P, which has no action on cold water is placed into a salt solution of Q, a brown solid R is...(Solved)
When a grey powder P, which has no action on cold water is placed into a salt solution of Q, a brown solid R is deposited.
The blue solution of Q, fades giving way to a green solution.
a) Name the type of reaction that takes place.
b) Identify solids P and R
c) Write an equation for the reaction leading to formation of the brown solid.
Date posted: September 19, 2019. Answers (1)
- A student dissolved some ammonium nitrate salt in water in a glass beaker. The solution formed felt very cold.(Solved)
A student dissolved some ammonium nitrate salt in water in a glass beaker. The solution formed felt very cold.
a) Explain why the temperature of the resultant solution dropped
b) Represent the above information on an energy level diagram
c) What general name is given to such reactions
Date posted: September 19, 2019. Answers (1)
- Methane gas reacts with chlorine gas as shown in the equation below.(Solved)
Methane gas reacts with chlorine gas as shown in the equation below.

Use the bond energies in the table below to calculate the enthalpy for the above reaction.

Date posted: September 19, 2019. Answers (1)
- The set- up below was used to investigate the rate of diffusion of different gases.(Solved)
The set- up below was used to investigate the rate of diffusion of different gases.
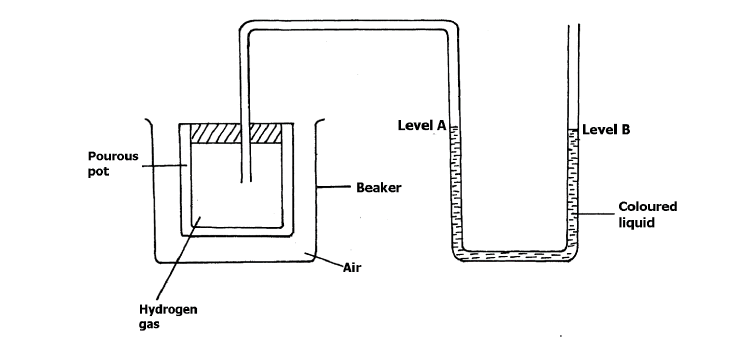
a) Explain why a coloured liquid is used in this experiment.
b) State and explain the observation made after 20 minutes.
Date posted: September 19, 2019. Answers (1)