i) Increase in temperature favours the backward reaction. that is, the equilibrium shifts to the left. Backward reaction is Endothermic.
ii) Equilibrium will shift to the left when the pressure is decreased and to the right when the pressure is increased.
Increase in pressure favours the side with few molecules and vise versa.
maurice.mutuku answered the question on September 25, 2019 at 05:59
- The volume of a given mass of a gas is 250cm3 at 270C and 720mmHg pressure. What will be the volume of the gas at...(Solved)
The volume of a given mass of a gas is 250cm3 at 270C and 720mmHg pressure. What will be the volume of the gas at s.t.p.
Date posted: September 25, 2019. Answers (1)
- The diagram below represents the structure of aluminium chloride.(Solved)
The diagram below represents the structure of aluminium chloride.

(i) Identify the bonds labelled M and N
(ii) What is the difference between bonds M and N?
Date posted: September 25, 2019. Answers (1)
- When solid M is dissolved in water, it dissolves to form a blue solution. Addition of ammonia solution forms a blue precipitate which dissolves in...(Solved)
When solid M is dissolved in water, it dissolves to form a blue solution. Addition of ammonia solution forms a blue precipitate which dissolves in excess to form a deep blue solution. Write the formula and name of the ion responsible for the deep blue solution.
Date posted: September 25, 2019. Answers (1)
- On combustion of 0.5g of an organic compound P (containing only carbon, hydrogen and oxygen),0.733g of carbon (IV) oxide and 0.3g of water were produced.
Determine...(Solved)
On combustion of 0.5g of an organic compound P (containing only carbon, hydrogen and oxygen),0.733g of carbon (IV) oxide and 0.3g of water were produced.
Determine the empirical formula of P.
Date posted: September 25, 2019. Answers (1)
- Radioactive polonium -216 decays as shown below.(Solved)
Radioactive polonium -216 decays as shown below.

(i) Find the value of M and N.
(ii) If after 112 days 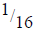 of Polonium remained, calculate the half-life of polonium.
of Polonium remained, calculate the half-life of polonium.
Date posted: September 25, 2019. Answers (1)
- Zinc metal can be used in the laboratory to prepare hydrogen gas from an appropriate mineral acid while copper metal cannot.
Explain.(Solved)
Zinc metal can be used in the laboratory to prepare hydrogen gas from an appropriate mineral acid while copper metal cannot.
Explain.
Date posted: September 24, 2019. Answers (1)
- How would the following pair of compounds be chemically distinguished? CH3COOH and CH3CH2OH.(Solved)
How would the following pair of compounds be chemically distinguished? CH3COOH and CH3CH2OH.
Date posted: September 24, 2019. Answers (1)
- Differentiate elaborately between Elements and Compounds(Solved)
Differentiate elaborately between Elements and Compounds.
Date posted: September 23, 2019. Answers (1)
- Calculate the number of Calcium atoms in 10g of calcium.
(Ca = 40, Avogadro number = 6.0 x 1023)(Solved)
Calculate the number of Calcium atoms in 10g of calcium.
(Ca = 40, Avogadro number = 6.0 x 1023)
Date posted: September 23, 2019. Answers (1)
- Your friend’s clothes have caught fire. In order to extinguish the fire you decide to cover with a damp blanket. (Solved)
Your friend’s clothes have caught fire. In order to extinguish the fire you decide to cover with a damp blanket. What is the purpose of the damp blanket?
Date posted: September 23, 2019. Answers (1)
- The flow chart below shows a series of reactions starting withpropan-l-ol. Study it and answer the questions that follow.(Solved)
The flow chart below shows a series of reactions starting withpropan-l-ol. Study it and answer the questions that follow.

(i) Name:
I. Process Y
II. Substances R and C
III. Process W
IV. Polymer X
(ii) Write a balanced chemical equation for the combustion of propane.
(iii) State one use of polymer X
Date posted: September 23, 2019. Answers (1)
- State how burning can be used to distinguish between prepane and propyne. Explain your answer.(Solved)
(a) State how burning can be used to distinguish between prepane and propyne. Explain your answer.
(b) Draw the structural formular of the 4th member of the homologous series in which propyne is a member
Date posted: September 23, 2019. Answers (1)
- Biogas is made from natural organic waste. A from four student set –up the apparatus below to investigate the composition of the gas. He passed...(Solved)
Biogas is made from natural organic waste. A from four student set –up the apparatus below to investigate the composition of the gas. He passed it over heated copper (II) oxide. After that he obtained two products. One was cooled in a boiling tube while the other was collected using a syringe.

(a) What is the function of calcium oxide
(b) Name product
i) P
ii) Y
(c) Give the two observations made in tube X
(d) i) Name the type of reaction taking place in tube X
ii) Write the equation for the reaction above in (d)(i)
(e) What elements are present in biogas
(f) Explain why one tube in boiling tube Q is longer than the other
Date posted: September 23, 2019. Answers (1)
- Using the following thermochemical equations draw an energy cycle diagram.(Solved)
Using the following thermochemical equations draw an energy cycle diagram.

(i) Name the heat of change represented by thermochemical equation A
(ii) Using thermochemical equations A, B, C. Calculate the molar heat of combustion of ethyne
(iii) Give 3 factors considered for choosing a good fuel
Date posted: September 23, 2019. Answers (1)
- The following are half-cell reactions and their reduction potentials
(i) Write the cell representation for the electrochemical cell that would give the highest E? value.
(ii)...(Solved)
The following are half-cell reactions and their reduction potentials

(i) Write the cell representation for the electrochemical cell that would give the highest Eθ value.
(ii) Give one industrial use of electrolysis
Date posted: September 23, 2019. Answers (1)
- Study the diagrams below.(Solved)
Study the diagrams below.

(a) State the observations made at I and II
(b) Write the equation to show the reactions at II if dry sulphur (IV) oxide was used in place of dry chlorine.
Date posted: September 20, 2019. Answers (1)
- Study the flow chart below and answer the questions that follow(Solved)
Study the flow chart below and answer the questions that follow

Process Y
i. Write the formula of Alcohol X, Compound Z and name process Y.
ii. Propane and Chlorine react as shown below :

a) Name the type of reaction that takes place.
b) State the condition under which this reaction takes place.
Date posted: September 20, 2019. Answers (1)
- Study the table below and answer the questions that follow(Solved)
Study the table below and answer the questions that follow

i) What name is given to a series of organic compounds like the ones in the table?
ii) To what class of organic compounds does the above hydrocarbon belongs?
iii) Select one hydrocarbon that would be a gas at room temperature (298K) given a reason for your answer.
iv) Give the formula of the seventh member of the above series.
v) What is the relationship between the boiling point and the relative molecular masses of the hydrocarbons in the table above?
Explain your answer.
Date posted: September 20, 2019. Answers (1)
- Study the scheme below and answer questions that follow.(Solved)
Study the scheme below and answer questions that follow.

i) Name
Gas K
Gas M
ii) State observation made in
Step I
Step II
iii) State the conditions necessary for step II to occur.
iv) Write an equation to show how pollution effect of sulphur (IV) oxide is controlled in contact process.
v) Explain the role of sulphur in vulcanization of rubber.
Date posted: September 20, 2019. Answers (1)
- Study the structure below and answer questions that follow.(Solved)
Study the structure below and answer questions that follow:

a) What observation is made when the molecule above is heated to a temperature of 1130 C
b) Write an equation for the reaction of atom of the above structure with hydrogen.
Date posted: September 20, 2019. Answers (1)