- The chromatogram below shows the constituents of a flower extract. Study it and answer the question that follows.(Solved)
The chromatogram below shows the constituents of a flower extract. Study it and answer the question that follows.

Give a reason to explain the different positions of red and yellow pigments.
Date posted: September 25, 2019. Answers (1)
- A mixture of substance K (density 0.626g/cm3) and Z (density 0.85g/cm3) was allowed to settle in a container as shown below.(Solved)
A mixture of substance K (density 0.626g/cm3) and Z (density 0.85g/cm3) was allowed to settle in a container as shown below.

a) Which liquid forms layer (I)
b) Explain your answer in (a) above.
Date posted: September 25, 2019. Answers (1)
- The table below is a part of the periodic table. The letters are not the actual symbols of the element. Study it and answer the...(Solved)
The table below is a part of the periodic table. The letters are not the actual symbols of the element. Study it and answer the questions that follow.

a) Select an element which is the most reactive.
b) How do the ionic radius of T and S compare? Explain.
Date posted: September 25, 2019. Answers (1)
- Half-life of a radio-active elements is 30 days. Calculate the time required for its activity 37.5 counts per minute.(Solved)
Half-life of a radio-active elements is 30 days. Calculate the time required for its activity 37.5 counts per minute.
Date posted: September 25, 2019. Answers (1)
- During an electrolysis of Zinc sulphate using inert electrodes, a current of 0.5A was passed for 40 minutes on a steady current.
a) Write down the...(Solved)
During an electrolysis of Zinc sulphate using inert electrodes, a current of 0.5A was passed for 40 minutes on a steady current.
a) Write down the equation at the cathode.
b) Calculate the volume produced at the cathode given that 1F = 96,500c, MGV = 22.4 litres.
Date posted: September 25, 2019. Answers (1)
- The diagram below was used to prepare and collect Sulphur (IV) oxide gas.(Solved)
The diagram below was used to prepare and collect Sulphur (IV) oxide gas.

a) Identify solid M.
b) State two properties of SO2 that makes possible to be collected in the method shown.
c) What are the optimum conditions of conversion of SO2 to SO3.
Date posted: September 25, 2019. Answers (1)
- Study the flow chart given below.(Solved)
Study the flow chart given below.

a) Name substance B.
b) Give one us of product C.
c) Write the equation between substance B to form substance C.
Date posted: September 25, 2019. Answers (1)
- The table below shows the electrical conductivity of substances A, B and C.(Solved)
The table below shows the electrical conductivity of substances A, B and C.

a) Give the type of structure and bonding that is present in substance A.
b) Which substance is likely to be sodium chloride. Explain.
c) Which one of the substance is likely to be plastic?
i) Explain why the substance you have given in (c) above behaves in the way it does
d) Give the type of structure and bonding that is present in substance A
Date posted: September 25, 2019. Answers (1)
- A student used the set-up shown in the diagram below in order to study the reactions of some metals with steam. The experiment was carried...(Solved)
A student used the set-up shown in the diagram below in order to study the reactions of some metals with steam. The experiment was carried out for ten minutes.

a) What observation would be made if gas H was ignited.
b) When the experiment was repeated using lead powder instead of Beryllium very little of gas H was obtained. Give a reason for this observation.
c) Name another gas which is used together with hydrogen in welding.
Date posted: September 25, 2019. Answers (1)
- Study the flow chart below and answer the question that follows.(Solved)
Study the flow chart below and answer the question that follows.

Identify
i) Solid V
ii) Solution R
iii) Solution Q
Date posted: September 25, 2019. Answers (1)
- Calculate the maximum volume of oxygen, measured at s.t.p., that can be obtained by heating a solution
containing 8.8g of hydrogen peroxide.(Solved)
Calculate the maximum volume of oxygen, measured at s.t.p., that can be obtained by heating a solution
containing 8.8g of hydrogen peroxide.
Date posted: September 25, 2019. Answers (1)
- A compound contains 29.1% sodium, 40.5% sulphur and the rest is oxygen. Find the empirical formulae.
(Na = 23, S = 32, O = 16)(Solved)
A compound contains 29.1% sodium, 40.5% sulphur and the rest is oxygen. Find the empirical formulae.
(Na = 23, S = 32, O = 16)
Date posted: September 25, 2019. Answers (1)
- Draw an Isomer of Pentene.(Solved)
Draw an Isomer of Pentene.
Date posted: September 25, 2019. Answers (1)
- Determine the volume of 2.0M NaOH which when diluted to 250cm3 would produce a 0.8M NaOH solution.(Solved)
Determine the volume of 2.0M NaOH which when diluted to 250cm3 would produce a 0.8M NaOH solution.
Date posted: September 25, 2019. Answers (1)
- Explain what happens to the position of the equilibrium when:
i) Temperature is raised.
ii) Pressure is changed.(Solved)
Given the equation 
Explain what happens to the position of the equilibrium when:
i) Temperature is raised.
ii) Pressure is changed.
Date posted: September 25, 2019. Answers (1)
- The volume of a given mass of a gas is 250cm3 at 270C and 720mmHg pressure. What will be the volume of the gas at...(Solved)
The volume of a given mass of a gas is 250cm3 at 270C and 720mmHg pressure. What will be the volume of the gas at s.t.p.
Date posted: September 25, 2019. Answers (1)
- The diagram below represents the structure of aluminium chloride.(Solved)
The diagram below represents the structure of aluminium chloride.

(i) Identify the bonds labelled M and N
(ii) What is the difference between bonds M and N?
Date posted: September 25, 2019. Answers (1)
- When solid M is dissolved in water, it dissolves to form a blue solution. Addition of ammonia solution forms a blue precipitate which dissolves in...(Solved)
When solid M is dissolved in water, it dissolves to form a blue solution. Addition of ammonia solution forms a blue precipitate which dissolves in excess to form a deep blue solution. Write the formula and name of the ion responsible for the deep blue solution.
Date posted: September 25, 2019. Answers (1)
- On combustion of 0.5g of an organic compound P (containing only carbon, hydrogen and oxygen),0.733g of carbon (IV) oxide and 0.3g of water were produced.
Determine...(Solved)
On combustion of 0.5g of an organic compound P (containing only carbon, hydrogen and oxygen),0.733g of carbon (IV) oxide and 0.3g of water were produced.
Determine the empirical formula of P.
Date posted: September 25, 2019. Answers (1)
- Radioactive polonium -216 decays as shown below.(Solved)
Radioactive polonium -216 decays as shown below.

(i) Find the value of M and N.
(ii) If after 112 days 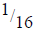 of Polonium remained, calculate the half-life of polonium.
of Polonium remained, calculate the half-life of polonium.
Date posted: September 25, 2019. Answers (1)