-
The chromatogram below shows the constituents of a flower extract. Study it and answer the question that follows.
(Solved)
The chromatogram below shows the constituents of a flower extract. Study it and answer the question that follows.

Give a reason to explain the different positions of red and yellow pigments.
Date posted:
September 25, 2019
.
Answers (1)
-
The diagram below was used to prepare and collect Sulphur (IV) oxide gas.
(Solved)
The diagram below was used to prepare and collect Sulphur (IV) oxide gas.

a) Identify solid M.
b) State two properties of SO2 that makes possible to be collected in the method shown.
c) What are the optimum conditions of conversion of SO2 to SO3.
Date posted:
September 25, 2019
.
Answers (1)
-
The table below shows the electrical conductivity of substances A, B and C.
(Solved)
The table below shows the electrical conductivity of substances A, B and C.

a) Give the type of structure and bonding that is present in substance A.
b) Which substance is likely to be sodium chloride. Explain.
c) Which one of the substance is likely to be plastic?
i) Explain why the substance you have given in (c) above behaves in the way it does
d) Give the type of structure and bonding that is present in substance A
Date posted:
September 25, 2019
.
Answers (1)
-
A student used the set-up shown in the diagram below in order to study the reactions of some metals with steam. The experiment was carried...
(Solved)
A student used the set-up shown in the diagram below in order to study the reactions of some metals with steam. The experiment was carried out for ten minutes.

a) What observation would be made if gas H was ignited.
b) When the experiment was repeated using lead powder instead of Beryllium very little of gas H was obtained. Give a reason for this observation.
c) Name another gas which is used together with hydrogen in welding.
Date posted:
September 25, 2019
.
Answers (1)
-
Draw an Isomer of Pentene.
(Solved)
Draw an Isomer of Pentene.
Date posted:
September 25, 2019
.
Answers (1)
-
Determine the volume of 2.0M NaOH which when diluted to 250cm3 would produce a 0.8M NaOH solution.
(Solved)
Determine the volume of 2.0M NaOH which when diluted to 250cm3 would produce a 0.8M NaOH solution.
Date posted:
September 25, 2019
.
Answers (1)
-
The diagram below represents the structure of aluminium chloride.
(Solved)
The diagram below represents the structure of aluminium chloride.

(i) Identify the bonds labelled M and N
(ii) What is the difference between bonds M and N?
Date posted:
September 25, 2019
.
Answers (1)
-
The flow chart below shows a series of reactions starting withpropan-l-ol. Study it and answer the questions that follow.
(Solved)
The flow chart below shows a series of reactions starting withpropan-l-ol. Study it and answer the questions that follow.

(i) Name:
I. Process Y
II. Substances R and C
III. Process W
IV. Polymer X
(ii) Write a balanced chemical equation for the combustion of propane.
(iii) State one use of polymer X
Date posted:
September 23, 2019
.
Answers (1)
-
Biogas is made from natural organic waste. A from four student set –up the apparatus below to investigate the composition of the gas. He passed...
(Solved)
Biogas is made from natural organic waste. A from four student set –up the apparatus below to investigate the composition of the gas. He passed it over heated copper (II) oxide. After that he obtained two products. One was cooled in a boiling tube while the other was collected using a syringe.

(a) What is the function of calcium oxide
(b) Name product
i) P
ii) Y
(c) Give the two observations made in tube X
(d) i) Name the type of reaction taking place in tube X
ii) Write the equation for the reaction above in (d)(i)
(e) What elements are present in biogas
(f) Explain why one tube in boiling tube Q is longer than the other
Date posted:
September 23, 2019
.
Answers (1)
-
Study the flow chart below and answer the questions that follow
(Solved)
Study the flow chart below and answer the questions that follow

Process Y
i. Write the formula of Alcohol X, Compound Z and name process Y.
ii. Propane and Chlorine react as shown below :

a) Name the type of reaction that takes place.
b) State the condition under which this reaction takes place.
Date posted:
September 20, 2019
.
Answers (1)
-
Equal masses of magnesium ribbon were reacted separately with equal volumes of 1M hydrochloric acid and 1M methanoic acid. The results were plotted on a...
(Solved)
Equal masses of magnesium ribbon were reacted separately with equal volumes of 1M hydrochloric acid and 1M methanoic acid. The results were plotted on a graph as shown below. Two curves X and Y were obtained.

a) Explain which curves represents:
i) 1M hydrochloric acid.
ii) 1M Methanoic acid.
b) State the significance of point Z.
c) Write ionic equation for the reaction between magnesium and dilute hydrochloric acid.
d) Calculate the maximum mass of the gas that would be liberated if 1.2g of magnesium reacted with excess hydrochloric acid.
(Mg = 24, H =1).
e) Calculate the volume of the gas produced in (e) above at r.t.p (molar gas volume at r.t.p) = 24dm3.
Date posted:
September 20, 2019
.
Answers (1)
-
The arrangements below shows a set-up to investigate the effect of an electric current on molten lead (II) iodide.
(Solved)
The arrangements below shows a set-up to investigate the effect of an electric current on molten lead (II) iodide.

a) Identify two mistakes in the set-up.
b) State three observations made after correcting the mistakes.
c) What particles are responsible for electrical conductivity?
d) Write the equations for the reactions taking place at the electrodes.
e) Indicate on the diagram direction of flow of electric current.
Date posted:
September 20, 2019
.
Answers (1)
-
Study the set-up of apparatus below and answer the questions that follow.
(Solved)
Study the set-up of apparatus below and answer the questions that follow.

a) State and explain the observation that would be made in tube L as the experiment progresses in the first few minutes.
Observation.
Explanation.
b) How would the observations in the tube L change if the experiment is carried out for a long time. Explain using a chemical equation.
Observation.
Equation.
c) State three observations made when liquid S is reacted with sodium metal.
d) State the use of the suction pump in this experiment.
Date posted:
September 19, 2019
.
Answers (1)
-
When solid Zinc carbonate was added to a solution of hydrogen chloride in methylbenzene, there was no observable change.On addition of some water to the...
(Solved)
When solid Zinc carbonate was added to a solution of hydrogen chloride in methylbenzene, there was no observable change.On addition of some water to the mixture there was effervescence. Explain these observations.
Date posted:
September 19, 2019
.
Answers (1)
-
The diagram below shows the heating curve of a pure substance. Study it and answer the questions that follow.
(Solved)
The diagram below shows the heating curve of a pure substance. Study it and answer the questions that follow.

(a) What are the physical states of the substances at points W and Y.
(b) Explain why the temperature remains constant between points B and C.
Date posted:
September 19, 2019
.
Answers (1)
-
Potassium manganate (VII) reacts with chloride salts to produce chlorine. Both chlorine and potassium manganate (VII) are strong oxidizing agents. Which one of the two...
(Solved)
Potassium manganate (VII) reacts with chloride salts to produce chlorine. Both chlorine and potassium manganate (VII) are strong oxidizing agents. Which one of the two is the stronger oxidizing agent? Explain your answer
Date posted:
September 19, 2019
.
Answers (1)
-
When a grey powder P, which has no action on cold water is placed into a salt solution of Q, a brown solid R is...
(Solved)
When a grey powder P, which has no action on cold water is placed into a salt solution of Q, a brown solid R is deposited.
The blue solution of Q, fades giving way to a green solution.
a) Name the type of reaction that takes place.
b) Identify solids P and R
c) Write an equation for the reaction leading to formation of the brown solid.
Date posted:
September 19, 2019
.
Answers (1)
-
Methane gas reacts with chlorine gas as shown in the equation below.
(Solved)
Methane gas reacts with chlorine gas as shown in the equation below.

Use the bond energies in the table below to calculate the enthalpy for the above reaction.

Date posted:
September 19, 2019
.
Answers (1)
-
The set- up below was used to investigate the rate of diffusion of different gases.
(Solved)
The set- up below was used to investigate the rate of diffusion of different gases.
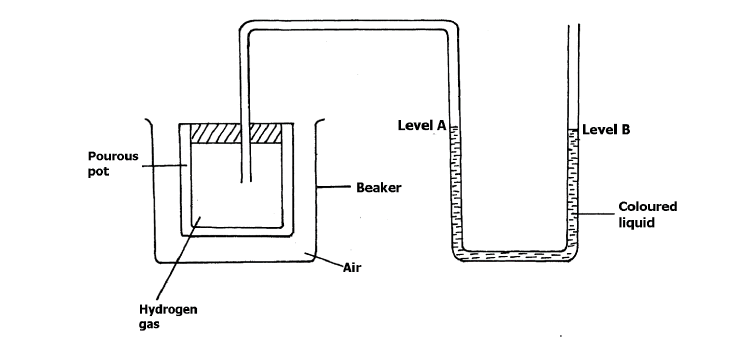
a) Explain why a coloured liquid is used in this experiment.
b) State and explain the observation made after 20 minutes.
Date posted:
September 19, 2019
.
Answers (1)
-
The table below gives information about some reactions of metals A,B, C and D and their rates.
(Solved)
The table below gives information about some reactions of metals A,B, C and D and their rates.
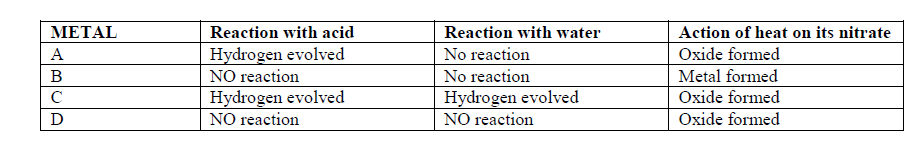
Arrange the metals in order of decreasing activity.
Date posted:
September 19, 2019
.
Answers (1)