- The figure below shows an inclined plane, a trolley of mass 30kg is pulled up a slope by a force of 100N, parallel to the...(Solved)
The figure below shows an inclined plane, a trolley of mass 30kg is pulled up a slope by a force of 100N, parallel to the slope. The trolley moves so that the centre of mass C travels from points A to B.
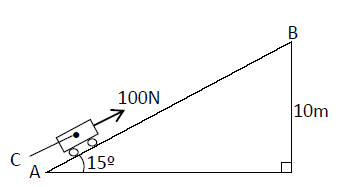
(i) What is the work done on the trolley against the gravitational force in moving from A to B.?
(ii) Determine the work done by the force in moving the trolley from A to B.
(iii) Determine the efficiency of the system.
(iv) Determine the work done in overcoming the frictional force.
(v) Determine the mechanical advantage of the system.
Date posted: September 26, 2019. Answers (1)
- The figure shows masses X, Y and Z placed at different points on a turn table. The turn table is rotated at different angular velocities.(Solved)
The figure shows masses X, Y and Z placed at different points on a turn table. The turn table is rotated at different angular velocities.
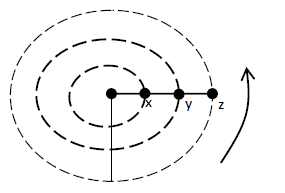
(i) State two factors that would cause the masses to slide.
(ii) At the time that start sliding off, state the mass with the highest angular velocity,give reason for your answer.
Date posted: September 26, 2019. Answers (1)
- In an experiment to determine the density of a liquid, the following readings were made.
Mass of empty density bottle = 20g
Mass of bottle filled with...(Solved)
In an experiment to determine the density of a liquid, the following readings were made.
Mass of empty density bottle = 20g
Mass of bottle filled with water = 70g
Mass of bottle filled with a liquid = 695g
a. Find the density of the liquid, given that density of water is 1000kgmˉ³.
b. Find the mass of the liquid.
Date posted: September 26, 2019. Answers (1)
- The figure below shows a uniform triangular lamina.Locate the centre of gravity of lamina.(Solved)
The figure below shows a uniform triangular lamina.Locate the centre of gravity of lamina.
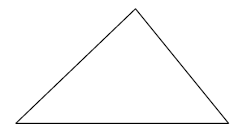
Date posted: September 26, 2019. Answers (1)
- A pulse-echo sounder is used by fishing boat to locate a shoal of fish in water. The sounder sends sound of frequency 21KHz and wavelength...(Solved)
A pulse-echo sounder is used by fishing boat to locate a shoal of fish in water. The sounder sends sound of frequency 21KHz and wavelength of 7.5cm. if the echo is received after 0.4seconds, determine how far the shoal of fish is from the base of the boat.
Date posted: September 26, 2019. Answers (1)
- Sketch a graph of displacement against time for a transverse wave of frequency of 50Hz of at least two cycles with amplitude 2cm.(Solved)
Sketch a graph of displacement against time for a transverse wave of frequency of 50Hz of at least two cycles with amplitude 2cm.
Date posted: September 26, 2019. Answers (1)
- When electromagnet radiation of wavelength 4.0x10-7 is incident on a metal surface, a stopping potential of 0.75V is just sufficient to prevent the emission photoelectrons....(Solved)
When electromagnet radiation of wavelength 4.0x10-7 is incident on a metal surface, a stopping potential of 0.75V is just sufficient to prevent the emission photoelectrons. determine the maximum kinetic energy of the emitted electrons when the stopping potential is zero
Date posted: September 26, 2019. Answers (1)
- Draw a sketch graph of stopping potential against frequency of incident radiation(Solved)
Draw a sketch graph of stopping potential against frequency of incident radiation
Date posted: September 26, 2019. Answers (1)
- Various isotopes of an element X can be distinguished by using the symbol what do the symbols A and Z? stand for.(Solved)
Various isotopes of an element X can be distinguished by using the symbol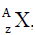 what do the symbols A and Z? stand for.
what do the symbols A and Z? stand for.
Date posted: September 26, 2019. Answers (1)
- Complete the ray diagram below and state one characteristic of the image formed by the following convex lens.(Solved)
Complete the ray diagram below and state one characteristic of the image formed by the following convex lens.
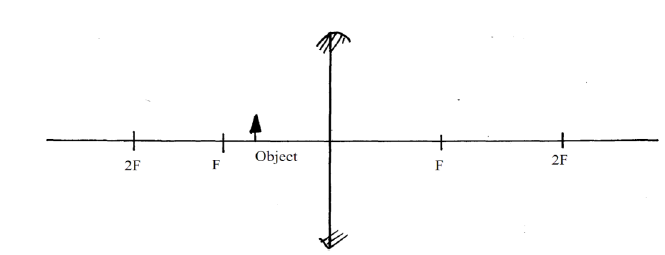
Date posted: September 26, 2019. Answers (1)
- Determine the angle of incidence and angle of reflection in the mirror shown below.(Solved)
Determine the angle of incidence and angle of reflection in the mirror shown below.

Date posted: September 26, 2019. Answers (1)
- You are provided with a long steel rod shown below.(Solved)
You are provided with a long steel rod shown below.

Using a diagram, describe how you would magnetise end A to obtain a south pole using an electric current.
Date posted: September 26, 2019. Answers (1)
- The figure below shows a CRO screen display trace when the Y-amplication control and time base setting are 100mV and 0.8ms/cm respectively.(Solved)
The figure below shows a CRO screen display trace when the Y-amplication control and time base setting are 100mV and 0.8ms/cm respectively.
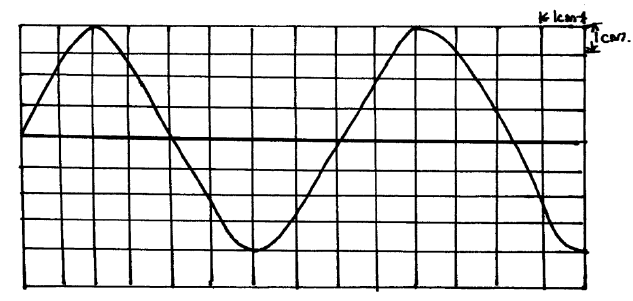
Calculate:
a) The peak potential difference.
b) The frequency of the signal.
Date posted: September 26, 2019. Answers (1)
- The figure below shows a circular conductor placed closely to a magnet. When the magnet is moved, a current is induced as shown. Indicate the...(Solved)
The figure below shows a circular conductor placed closely to a magnet. When the magnet is moved, a current is induced as shown. Indicate the direction of motion of the magnet.
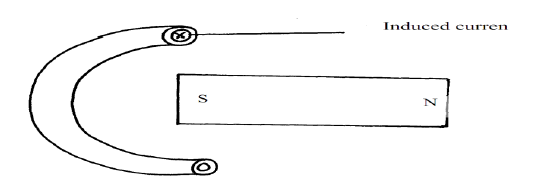
Date posted: September 26, 2019. Answers (1)
- Calculate the cost of using a electricity iron rated 1200W, for a total of 30hours given that the cost of electricity per KWh is ksh...(Solved)
Calculate the cost of using a electricity iron rated 1200W, for a total of 30hours given that the cost of electricity per KWh is ksh 8.
Date posted: September 26, 2019. Answers (1)
- The figure below sow a current carrying conductor passing between two cardboards. Show the direction of the deflection on each compass on the cardboard.(Solved)
The figure below sow a current carrying conductor passing between two cardboards. Show the direction of the deflection on each compass on the cardboard.
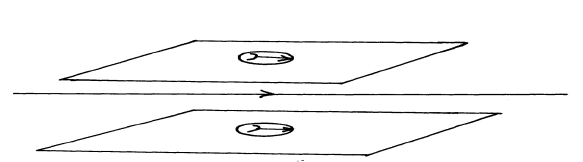
Date posted: September 26, 2019. Answers (1)
- A stone and a feather are dropped from rest from a building 20m tall. If they reach the ground at the same time, find.
(a) The...(Solved)
A stone and a feather are dropped from rest from a building 20m tall. If they reach the ground at the same time, find.
(a) The velocity with which they reach the ground. (Take g=l0m/s2)
(b) The condition under which they fall.
Date posted: September 26, 2019. Answers (1)
- Find the velocity ratio of the following gear wheels.(Solved)
Find the velocity ratio of the following gear wheels.

Date posted: September 26, 2019. Answers (1)
- The diagram below shows a metal tube made of iron and copper. The joint is tight at room temperature.(Solved)
The diagram below shows a metal tube made of iron and copper. The joint is tight at room temperature.

Explain how you would separate the two by changing the temperature given that copper expands more than iron for some change in temperature.
Date posted: September 26, 2019. Answers (1)
- A student used the measuring instrument shown below to measure the thickness of a cylindrical wire, If the wire is 10cm long, find the volume...(Solved)
A student used the measuring instrument shown below to measure the thickness of a cylindrical wire, If the wire is 10cm long, find the volume of the wire.

Date posted: September 26, 2019. Answers (1)