(i) Condenser
(ii) To indicate when a liquid is boiling, a thermometer reads a constant temperature
(iii) A
(iv) Ethanol
Reason:- It has a lower boiling of 78oC compared to water with a boiling point of 100oC
or - The liquid with the lower boiling point boils first and its vapours are condensed
and the condenser to be collected as the first distillate
(v) Fractional distillation
(vi) - To separate components of crude oil
- To isolate O2 and N2 from air
- To manufacture spirits
(vii)- They are immiscible liquids
- They have different but close boiling points
maurice.mutuku answered the question on October 11, 2019 at 09:41
-
State two criteria for determining the purity of a substance
(Solved)
State two criteria for determining the purity of a substance
Date posted:
October 11, 2019
.
Answers (1)
-
The apparatus below were used by a student to study the effect of heat on hydrated copper II sulphate
(Solved)
The apparatus below were used by a student to study the effect of heat on hydrated copper II sulphate
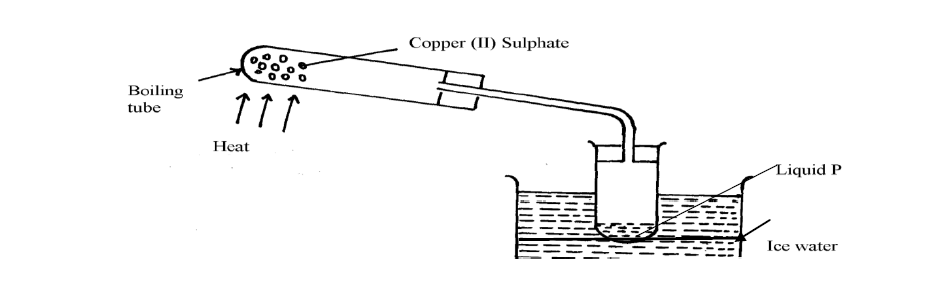
(a) What is the role of the ice cold water
(b) Name liquid P
(c) What observation is made in the boiling tube
Date posted:
October 11, 2019
.
Answers (1)
-
A form 1 student carried out the separation as shown in the set-up below:-
(Solved)
A form 1 student carried out the separation as shown in the set-up below:-
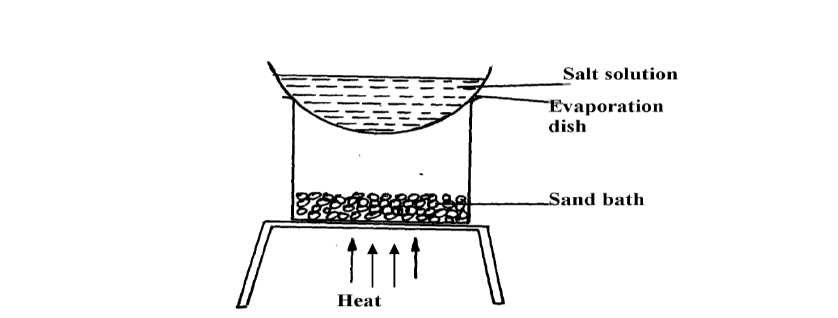
(i) Identify the method above.
(ii) Give one of its disadvantages
(iii) Name a mixture which can be separated by the set-up above
Date posted:
October 11, 2019
.
Answers (1)
-
Cooking oils comprise of a mixture of compounds which have a boiling point range
of 23oC to 27oC.
(i) What evidence is then to support the statement...
(Solved)
Cooking oils comprise of a mixture of compounds which have a boiling point range
of 23oC to 27oC.
(i) What evidence is there to support the statement that cooking oil is a mixture?
(ii)Name another experimental technique that could be used to confirm your answer in part (i) above
Date posted:
October 11, 2019
.
Answers (1)
-
Without using any laboratory chemical, describe a simple laboratory experiment to distinguish between calcium hydrogen carbonate and sodium hydrogen carbonate.
(Solved)
Without using any laboratory chemical, describe a simple laboratory experiment to distinguish between calcium hydrogen carbonate and sodium hydrogen carbonate.
Date posted:
October 11, 2019
.
Answers (1)
-
The two elements P and R were separately burned in air, the products gave the results recorded in the table below:
(Solved)
The two elements P and R were separately burned in air, the products gave the results recorded in the table below:

(a) Suggest the identity of element R.
(b) Describe how the nature of the solutions of the of the oxides were determined
Date posted:
October 11, 2019
.
Answers (1)
-
It is advisable to set a Bunsen burner to luminous flame prior to an experiment.
Explain
(Solved)
It is advisable to set a Bunsen burner to luminous flame prior to an experiment.
Explain
Date posted:
October 11, 2019
.
Answers (1)
-
The diagram below shows the heating curve of a pure substance. Study it and answer the
questions that follow:
(Solved)
The diagram below shows the heating curve of a pure substance. Study it and answer the
questions that follow:

(a) What physical changes are taking place at points X and Z?
(b)Explain what happens to the melting point of sodium chloride added to this substance
Date posted:
October 11, 2019
.
Answers (1)
-
The diagram below shows some parts of a Bunsen burner
(Solved)
The diagram below shows some parts of a Bunsen burner

Explain how the parts labelled T and U are suited to their functions
Date posted:
October 11, 2019
.
Answers (1)
-
The diagrams below are some common laboratory apparatus. Name each apparatus and state its use
(Solved)
The diagrams below are some common laboratory apparatus. Name each apparatus and state its use

Date posted:
October 11, 2019
.
Answers (1)
-
A mixture of hexane and water was shaken and left to separate as shown in the diagram below:
(Solved)
A mixture of hexane and water was shaken and left to separate as shown in the diagram below:

State the identity of;
(i) P
(ii) W
Date posted:
October 11, 2019
.
Answers (1)
-
The diagram below shows three methods for collecting gases in the laboratory
(Solved)
The diagram below shows three methods for collecting gases in the laboratory

(a) Name the methods A and B
(b) From the methods above, identify one that is suitable for collecting sulphur (IV) oxide.
Explain
Date posted:
October 11, 2019
.
Answers (1)
-
Draw a labelled diagram of a non-luminous flame
(Solved)
Draw a labelled diagram of a non-luminous flame
Date posted:
October 11, 2019
.
Answers (1)
-
Wooden splints F and G were placed in different zones of a Bunsen burner flame.
The diagram below gives the observations that were made
(Solved)
Wooden splints F and G were placed in different zones of a Bunsen burner flame.
The diagram below gives the observations that were made

(a) Explain the difference between F and G
(b) Name the type of flame that was used in the above experiment
Date posted:
October 11, 2019
.
Answers (1)
-
Calculate the number of Al3+ ions released when 30cm3 of 0.1M of Aluminum Sulphate is dissolved in water (L= 6.024 x 1023)
(Solved)


Date posted:
October 1, 2019
.
Answers (1)
-
The set-up below shows the products formed when solid lead (ii) nitrate is heated.
(Solved)
The set-up below shows the products formed when solid lead (ii) nitrate is heated.

a) Identify:
(i) Liquid X
(ii) Gas Y
b) When lead (ii) Nitrate crystals are heated, they decrepitate and decompose, what is meant by the term decrepitating?
Date posted:
October 1, 2019
.
Answers (1)
-
The following diagram shows the effect of electric current on lead (II) Chloride
(Solved)
The following diagram shows the effect of electric current on lead (II) Chloride.

a) When the circuit was completed no current flowed. Explain why.
b) When lead (II) Chloride was heated to about 300 degrees C, it melted and there was light on the bulb. State and explain the observation made at the anode.
Date posted:
October 1, 2019
.
Answers (1)
-
Explain using chemical means how you would differentiate between carbon (II) oxide and carbon (IV) oxide.
(Solved)
Explain using chemical means how you would differentiate between carbon (II) oxide and carbon (IV) oxide.
Date posted:
October 1, 2019
.
Answers (1)
-
The diagram below shows the effect of sunlight on chlorine water
(Solved)
The diagram below shows the effect of sunlight on chlorine water.

a) Identify gas W
b) Write an equation to show the formation of gas W
c) Which compound is left in the beaker after complete formation of gas W?
Date posted:
October 1, 2019
.
Answers (1)
-
Form two students from Anestar Premier High School reacted three elements as shown in the table below
(Solved)
Form two students from Anestar Premier High School reacted three elements as shown in the table below.

Which element (s) is likely to be:
i) Non-metal (s)
ii) Metal (s)
iii) Insoluble in water
Date posted:
October 1, 2019
.
Answers (1)