- Given a mixture of lead (II) oxide, ammonium chloride and sodium chloride, describe how this mixture can be separated to obtain a sample of each.(Solved)
Given a mixture of lead (II) oxide, ammonium chloride and sodium chloride, describe how this mixture can be separated to obtain a sample of each.
Date posted: October 11, 2019. Answers (1)
- Study the information below and answer the questions that follow:(Solved)
Study the information below and answer the questions that follow:

Describe how the mixture of solid R, S, and V can be separated
Date posted: October 11, 2019. Answers (1)
- The set-up below was used to separate a mixture:-(Solved)
The set-up below was used to separate a mixture:-
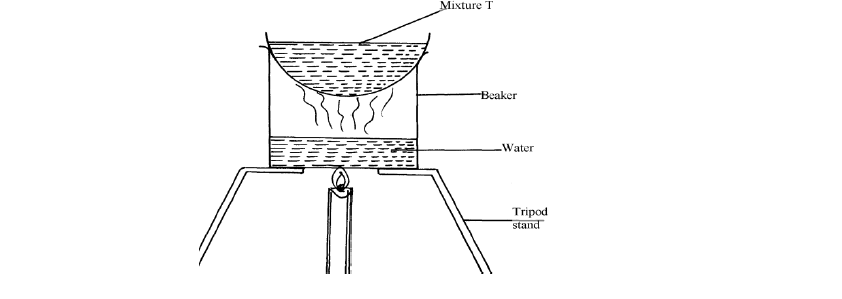
(a) Name the apparatus missing in the set-up
(b) Give one example of mixture T
(c) What is the name of this method of separation
Date posted: October 11, 2019. Answers (1)
- A student left some crushed fruit mixture with water for some days. He found the mixture had fermented. He concluded that the mixture was contaminated...(Solved)
A student left some crushed fruit mixture with water for some days. He found the mixture had fermented. He concluded that the mixture was contaminated with water and ethanol with boiling point of 100oC and 78oC respectively. The set-up of apparatus below are used to separate the mixture.
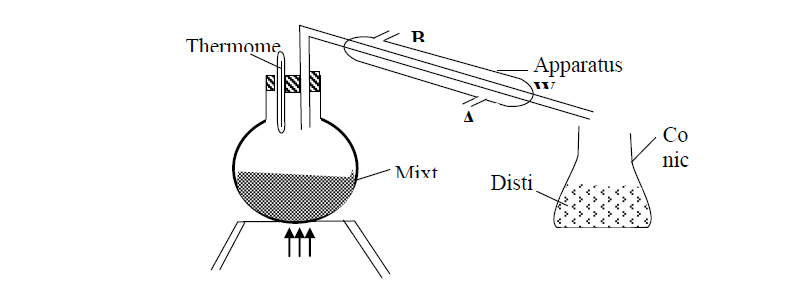
(i) Name the piece of apparatus labelled W
(ii) What is the purpose of the thermometer in the set-up?
iii) At which end of the apparatus W should tap water be connected?
(iv) Which liquid was collected as the first distillate? Explain
(v) What is the name given to the above method of separating mixture?
(vi) State two applications of the above method of separating mixtures
(vii) What properties of the mixture makes it possible for the component to be separated by the above methods?
Date posted: October 11, 2019. Answers (1)
- State two criteria for determining the purity of a substance(Solved)
State two criteria for determining the purity of a substance
Date posted: October 11, 2019. Answers (1)
- Classify the following processes as chemical changes or physical changes(Solved)
Classify the following processes as chemical changes or physical changes
Process physical or chemical
Neutralization ………………………………………
Sublimation ………………………………………
Fractional distillation ………………………………………..
Displacement reaction …………………………………………
Date posted: October 11, 2019. Answers (1)
- The apparatus below were used by a student to study the effect of heat on hydrated copper II sulphate(Solved)
The apparatus below were used by a student to study the effect of heat on hydrated copper II sulphate
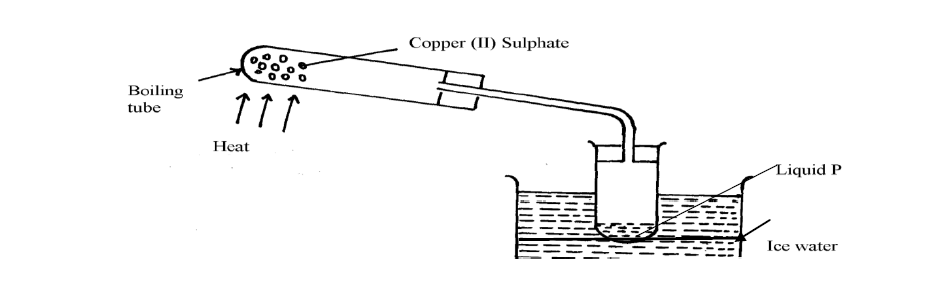
(a) What is the role of the ice cold water
(b) Name liquid P
(c) What observation is made in the boiling tube
Date posted: October 11, 2019. Answers (1)
- A form 1 student carried out the separation as shown in the set-up below:-(Solved)
A form 1 student carried out the separation as shown in the set-up below:-
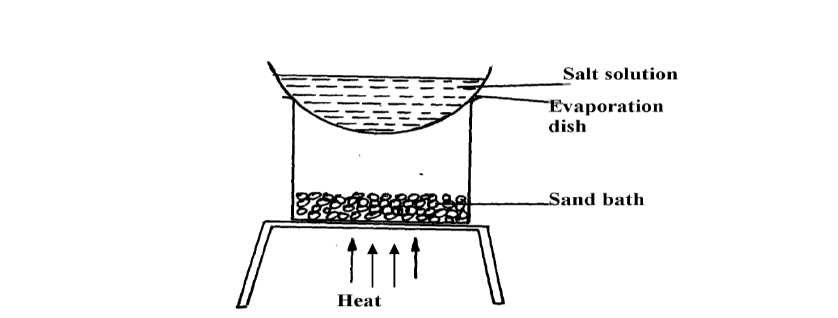
(i) Identify the method above.
(ii) Give one of its disadvantages
(iii) Name a mixture which can be separated by the set-up above
Date posted: October 11, 2019. Answers (1)
- Cooking oils comprise of a mixture of compounds which have a boiling point range
of 23oC to 27oC.
(i) What evidence is then to support the statement...(Solved)
Cooking oils comprise of a mixture of compounds which have a boiling point range
of 23oC to 27oC.
(i) What evidence is there to support the statement that cooking oil is a mixture?
(ii)Name another experimental technique that could be used to confirm your answer in part (i) above
Date posted: October 11, 2019. Answers (1)
- Without using any laboratory chemical, describe a simple laboratory experiment to distinguish between calcium hydrogen carbonate and sodium hydrogen carbonate.(Solved)
Without using any laboratory chemical, describe a simple laboratory experiment to distinguish between calcium hydrogen carbonate and sodium hydrogen carbonate.
Date posted: October 11, 2019. Answers (1)
- The two elements P and R were separately burned in air, the products gave the results recorded in the table below:(Solved)
The two elements P and R were separately burned in air, the products gave the results recorded in the table below:

(a) Suggest the identity of element R.
(b) Describe how the nature of the solutions of the of the oxides were determined
Date posted: October 11, 2019. Answers (1)
- It is advisable to set a Bunsen burner to luminous flame prior to an experiment.
Explain(Solved)
It is advisable to set a Bunsen burner to luminous flame prior to an experiment.
Explain
Date posted: October 11, 2019. Answers (1)
- The diagram below shows the heating curve of a pure substance. Study it and answer the
questions that follow:(Solved)
The diagram below shows the heating curve of a pure substance. Study it and answer the
questions that follow:

(a) What physical changes are taking place at points X and Z?
(b)Explain what happens to the melting point of sodium chloride added to this substance
Date posted: October 11, 2019. Answers (1)
- Study the information in the table below and answer questions that follow.
(Letters given are not real symbols)(Solved)
Study the information in the table below and answer questions that follow.
(Letters given are not real symbols)

Explain why the ionic radius of :-
(a) B+ is greater than that of A+
(b) C2+ is smaller than the of A+
Date posted: October 11, 2019. Answers (1)
- The diagram below shows some parts of a Bunsen burner(Solved)
The diagram below shows some parts of a Bunsen burner

Explain how the parts labelled T and U are suited to their functions
Date posted: October 11, 2019. Answers (1)
- The diagrams below are some common laboratory apparatus. Name each apparatus and state its use(Solved)
The diagrams below are some common laboratory apparatus. Name each apparatus and state its use

Date posted: October 11, 2019. Answers (1)
- A mixture of hexane and water was shaken and left to separate as shown in the diagram below:(Solved)
A mixture of hexane and water was shaken and left to separate as shown in the diagram below:

State the identity of;
(i) P
(ii) W
Date posted: October 11, 2019. Answers (1)
- The diagram below shows three methods for collecting gases in the laboratory(Solved)
The diagram below shows three methods for collecting gases in the laboratory

(a) Name the methods A and B
(b) From the methods above, identify one that is suitable for collecting sulphur (IV) oxide.
Explain
Date posted: October 11, 2019. Answers (1)
- Give two drugs that are commonly abused by the youth.(Solved)
Give two drugs that are commonly abused by the youth.
Date posted: October 11, 2019. Answers (1)
- What is a drug?(Solved)
What is a drug?
Date posted: October 11, 2019. Answers (1)