a) Methyl Orange-Red/Pink
Phenolphthalein-Colourless/Pink
b) The PH of 0.1M KOH is higher than of 0.1M aqueous ammonia KOH is strongly dissociated in solution.
maurice.mutuku answered the question on October 14, 2019 at 04:58
-
Below are the pH values of 4 types of medicine represented by letters P, Q, R and S
(Solved)
Below are the pH values of 4 types of medicine represented by letters P, Q, R and S
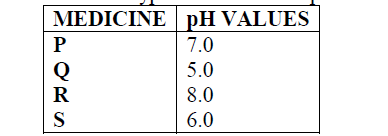
a) It is not advisable to use S when a patient has indigestion .Explain
b) What is the role of chemistry in drug manufacture
Date posted:
October 11, 2019
.
Answers (1)
-
During the electrolysis of dilute copper {II} chloride using carbon electrodes, a current of 1.5A was passed through the solution for 2 hours and 30...
(Solved)
During the electrolysis of dilute copper {II} chloride using carbon electrodes, a current of 1.5A was passed through the solution for 2 hours and 30 minutes.
[a] Write the ionic equation of the reaction that occurred at the cathode.
b] Given R.A.M of copper = 64 and 1F = 96500C, calculate the change in mass of the cathode.
Date posted:
October 11, 2019
.
Answers (1)
-
A reaction can be represented as;
(Solved)
A reaction can be represented as;
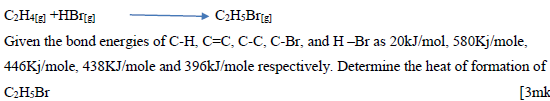
Date posted:
October 11, 2019
.
Answers (1)
-
A polymer can be represented as follows
(Solved)
A polymer can be represented as follows

[a] Name and draw the structure of the monomer.
[b] What type of polymerization occurs in the above case?
[c] Given that the molecular mass of the polymer is 25620, how many units of the monomer make the polymer.
Date posted:
October 11, 2019
.
Answers (1)
-
At 600C, 38 grams of lead{II} nitrate saturate 56cm3 of water. Determine the solubility of lead {II} nitrate at this temperature.
(Solved)
At 600C, 38 grams of lead{II} nitrate saturate 56cm3 of water. Determine the solubility of lead {II} nitrate at this temperature.
Date posted:
October 11, 2019
.
Answers (1)
-
Rusting is a process that causes massive destruction of iron structures
[a] State one condition that accelerates rusting.
[b] State one advantage of rusting
(Solved)
Rusting is a process that causes massive destruction of iron structures.
[a] State one condition that accelerates rusting.
[b] State one advantage of rusting.
Date posted:
October 11, 2019
.
Answers (1)
-
Solid copper (II) oxide is a base although it does not turn litmus paper to blue. Explain
(Solved)
Solid copper (II) oxide is a base although it does not turn litmus paper to blue. Explain
Date posted:
October 11, 2019
.
Answers (1)
-
When sodium nitrate was heated a solid A and gas B were produced identify solid A and gas B.
(Solved)
When sodium nitrate was heated a solid A and gas B were produced identify solid A and gas B.
Date posted:
October 11, 2019
.
Answers (1)
-
Give one example of an acid salt.
(Solved)
Give one example of an acid salt .
Date posted:
October 11, 2019
.
Answers (1)
-
The reduction potential of elements K, L, M, and P are as given below.
(Solved)
The reduction potential of elements K, L, M, and P are as given below.
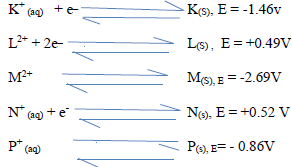
(i) Which letter represents the, strongest reducing agent? give a reason.
(ii) Which two letters represent elements whose half cells would form an electro chemical cell with the largest e.m.f?.
(iii) Calculate the e.m.f of the cell formed in (ii) above.
(d) During the electrolysis of a molten chloride of metal Q, a current of 0.25A was passed though the molten chloride for 2 hours and 10minutes. Given that 0.9grams of metal Q were deposited at the cathode.
(i) Calculate the quantity of electricity passed.
(ii) Charge carried by the ions of metal Q given that R.A.M of metal Q is 84.
Date posted:
October 11, 2019
.
Answers (1)
-
(a) Calamine is one of the ores from which zinc can be extracted from
(Solved)
(a) Calamine is one of the ores from which zinc can be extracted from.
(i) Name any other ore from which zinc can be extracted from.
(ii) The calamine is usually decomposed by heating to obtain substance M as shown below.

Identify substance M.
(iii) Identify two methods that can be used to obtain zinc from substance M
(b) During the extraction of zinc, name two gases likely to emitted into the air and that are likely to cause pollution
(c) State one likely pollution effects of each of the gases you have mentioned in (a) above
(d) State one possible use of zinc metal
Date posted:
October 11, 2019
.
Answers (1)
-
The information below gives PH values of solutions V, W, X, Y Z
(Solved)
The information below gives PH values of solutions V, W, X, Y Z

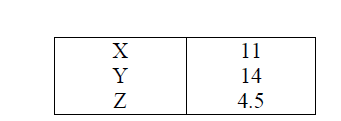
(a) Which solution is likely to be:
(i) Calcium hydroxide?
(ii) Rain water?
(b) Which solution would react most vigorously with Zinc carbonate
Date posted:
October 11, 2019
.
Answers (1)
-
The diagram below shows the contact process used in the manufacture of concentrated sulphuric(vi) acid.
(Solved)
The diagram below shows the contact process used in the manufacture of concentrated sulphuric(vi) acid.
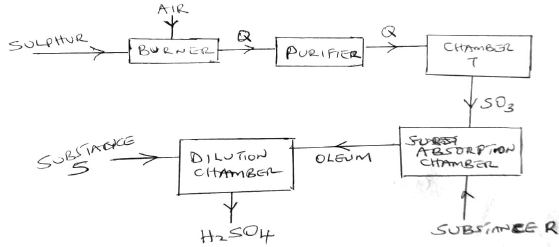
a) Identify the following:
i) Substance Q formed in the burner
ii) Chamber T
iii) Substance R
iv) Substance S
ii) Write the chemical equation occurring in the dilution chamber.
iii) Why is it necessary to pass substance Q though a purifier.
iv) State one use of sulphuric (VI) acid.
Date posted:
October 11, 2019
.
Answers (1)
-
The diagram below shows the process used to obtain Sulphur from underground deposits.
(Solved)
The diagram below shows the process used to obtain Sulphur from underground deposits.
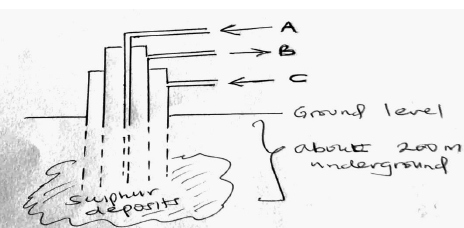
i) Name the above process used to obtain sulphur from the underground deposits
ii) Name the substance passed through pipe
A
B
iii) State two properties of Sulphur that makes it possible to extract using the above process.
Date posted:
October 11, 2019
.
Answers (1)
-
The table below shows solutions A, B and C are tested and observations records as shown:
(Solved)
The table below shows solutions A, B and C are tested and observations records as shown:

(a) Using the table above, name an acid
(b) How does the pH value of 1M potassium hydroxide solution compare with that of
1M aqueous ammonia? Explain
Date posted:
October 11, 2019
.
Answers (1)
-
a)Name two apparatuse that can be used for determining mass in a laboratory.
(Solved)
a)Name two apparatus that can be used for determining mass in a laboratory.
(b) One of the flames produced by Bunsen burner is the luminous flame.
i) Explain why this flame is very bright
ii) State two disadvantages of the luminous flame.
(c) Air is usually one of the substances that is considered as a mixture.
(i) Identify the two most abundant component of air.
(ii) Give two reasons why the air is considered as a mixture.
(iii) One of the components of air is carbon (iv) oxide. Describe an experiment that can be used to prove the presence of carbon (iv) oxide in the air.
Date posted:
October 11, 2019
.
Answers (1)
-
Name the following compounds
(i) CH3CH2CH2COOH
(Solved)
Name the following compounds
(i) CH3CH2CH2COOH
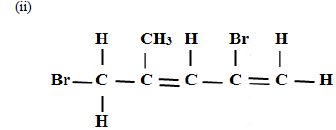
(iii)CH3CH2OOCCH2CH3
b) Two types of detergents P and Q can be represented as
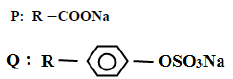
(i) Identify each type of the detergent.
(ii) Which of the two detergents is the best to use with hard water? Give a reason.
(iii) State one advantage of detergent P.
(iv) State one disadvantage of detergent Q.
(c) An hydrocarbon can be represented as follows.
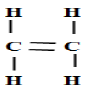
(i) Identify the hydrocarbon
(ii) Name two reagents that can reacted together to generate the hydrocarbon
Date posted:
October 11, 2019
.
Answers (1)
-
The setup below was used to separate two miscible liquids Q and T
(Boling points; Q =98° C, T=78°C)
(Solved)
The setup below was used to separate two miscible liquids Q and T
(Boling points; Q =98° C, T=78°C)
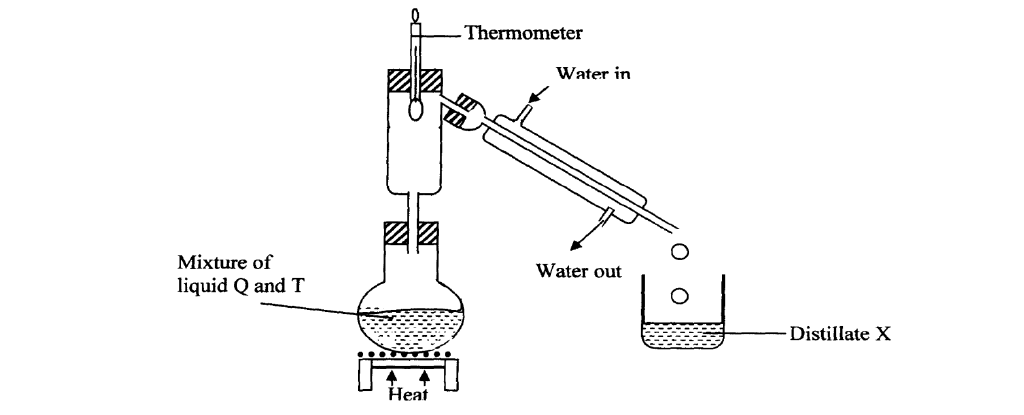
(a) Identify the mistakes in the setup above
(b)Identify Distillate X
Date posted:
October 11, 2019
.
Answers (1)
-
The grid below shows a section of the periodic table, the letters are not the actual chemical symbol
(Solved)
The grid below shows a section of the periodic table, the letters are not the actual chemical symbol
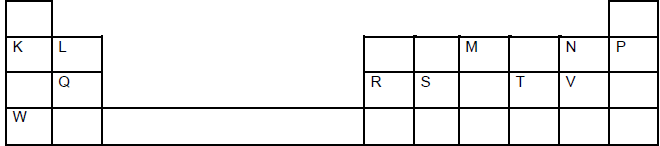
a) Name the family into which element P belongs to.
b) Which two elements forms the most soluble carbonates
c) With a reason, identify elements in period 3 with the largest atomic radius
d) Write the formula of the compound formed between Q and M
e) State two uses of element R and for each use , state property of element R that makes lts possible for the use
(i) Use
Property
(ii) Use
Property
f) Using dots and cross ,show bonding in the compound formed between R and oxygen.
g) In terms of structure and bonding explain why the oxides of element Thas relatively low boiling points
Date posted:
October 11, 2019
.
Answers (1)
-
Given a mixture of lead (II) oxide, ammonium chloride and sodium chloride, describe how this mixture can be separated to obtain a sample of each.
(Solved)
Given a mixture of lead (II) oxide, ammonium chloride and sodium chloride, describe how this mixture can be separated to obtain a sample of each.
Date posted:
October 11, 2019
.
Answers (1)