(a) Alkali is soluble base.
(b) Because it is lighter than air.
maurice.mutuku answered the question on October 14, 2019 at 05:21
-
The following data gives the pH values of some solutions
(Solved)
The following data gives the pH values of some solutions

(a) What colour change would occur in solution P on addition of two drops of phenolphthalein indicator?
(b) State the pH value of a resulting solution when equal moles of solution P and R react
Date posted:
October 14, 2019
.
Answers (1)
-
Explain why very little Carbon (IV) oxide gas is evolved when dilute sulphuric (VI) acid is added to lead (II) carbonate
(Solved)
Explain why very little Carbon (IV) oxide gas is evolved when dilute sulphuric (VI) acid is added to lead (II) carbonate
Date posted:
October 14, 2019
.
Answers (1)
-
Below are the pH values of 4 types of medicine represented by letters P, Q, R and S
(Solved)
Below are the pH values of 4 types of medicine represented by letters P, Q, R and S
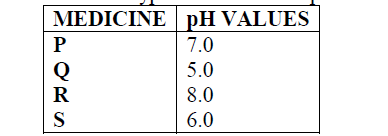
a) It is not advisable to use S when a patient has indigestion .Explain
b) What is the role of chemistry in drug manufacture
Date posted:
October 11, 2019
.
Answers (1)
-
During the electrolysis of dilute copper {II} chloride using carbon electrodes, a current of 1.5A was passed through the solution for 2 hours and 30...
(Solved)
During the electrolysis of dilute copper {II} chloride using carbon electrodes, a current of 1.5A was passed through the solution for 2 hours and 30 minutes.
[a] Write the ionic equation of the reaction that occurred at the cathode.
b] Given R.A.M of copper = 64 and 1F = 96500C, calculate the change in mass of the cathode.
Date posted:
October 11, 2019
.
Answers (1)
-
At 600C, 38 grams of lead{II} nitrate saturate 56cm3 of water. Determine the solubility of lead {II} nitrate at this temperature.
(Solved)
At 600C, 38 grams of lead{II} nitrate saturate 56cm3 of water. Determine the solubility of lead {II} nitrate at this temperature.
Date posted:
October 11, 2019
.
Answers (1)
-
Solid copper (II) oxide is a base although it does not turn litmus paper to blue. Explain
(Solved)
Solid copper (II) oxide is a base although it does not turn litmus paper to blue. Explain
Date posted:
October 11, 2019
.
Answers (1)
-
When sodium nitrate was heated a solid A and gas B were produced identify solid A and gas B.
(Solved)
When sodium nitrate was heated a solid A and gas B were produced identify solid A and gas B.
Date posted:
October 11, 2019
.
Answers (1)
-
The information below gives PH values of solutions V, W, X, Y Z
(Solved)
The information below gives PH values of solutions V, W, X, Y Z

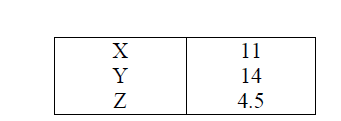
(a) Which solution is likely to be:
(i) Calcium hydroxide?
(ii) Rain water?
(b) Which solution would react most vigorously with Zinc carbonate
Date posted:
October 11, 2019
.
Answers (1)
-
The diagram below shows the contact process used in the manufacture of concentrated sulphuric(vi) acid.
(Solved)
The diagram below shows the contact process used in the manufacture of concentrated sulphuric(vi) acid.
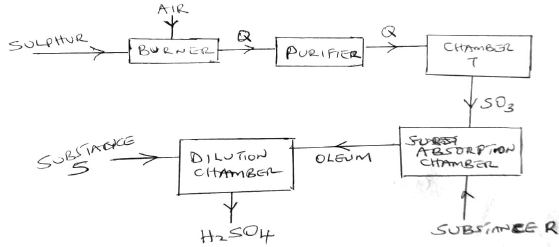
a) Identify the following:
i) Substance Q formed in the burner
ii) Chamber T
iii) Substance R
iv) Substance S
ii) Write the chemical equation occurring in the dilution chamber.
iii) Why is it necessary to pass substance Q though a purifier.
iv) State one use of sulphuric (VI) acid.
Date posted:
October 11, 2019
.
Answers (1)
-
The diagram below shows the process used to obtain Sulphur from underground deposits.
(Solved)
The diagram below shows the process used to obtain Sulphur from underground deposits.
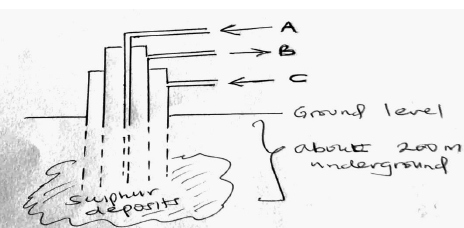
i) Name the above process used to obtain sulphur from the underground deposits
ii) Name the substance passed through pipe
A
B
iii) State two properties of Sulphur that makes it possible to extract using the above process.
Date posted:
October 11, 2019
.
Answers (1)
-
Name the following compounds
(i) CH3CH2CH2COOH
(Solved)
Name the following compounds
(i) CH3CH2CH2COOH
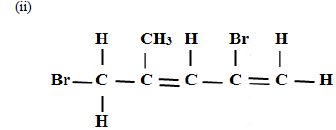
(iii)CH3CH2OOCCH2CH3
b) Two types of detergents P and Q can be represented as
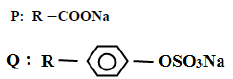
(i) Identify each type of the detergent.
(ii) Which of the two detergents is the best to use with hard water? Give a reason.
(iii) State one advantage of detergent P.
(iv) State one disadvantage of detergent Q.
(c) An hydrocarbon can be represented as follows.
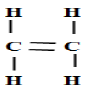
(i) Identify the hydrocarbon
(ii) Name two reagents that can reacted together to generate the hydrocarbon
Date posted:
October 11, 2019
.
Answers (1)
-
The setup below was used to separate two miscible liquids Q and T
(Boling points; Q =98° C, T=78°C)
(Solved)
The setup below was used to separate two miscible liquids Q and T
(Boling points; Q =98° C, T=78°C)
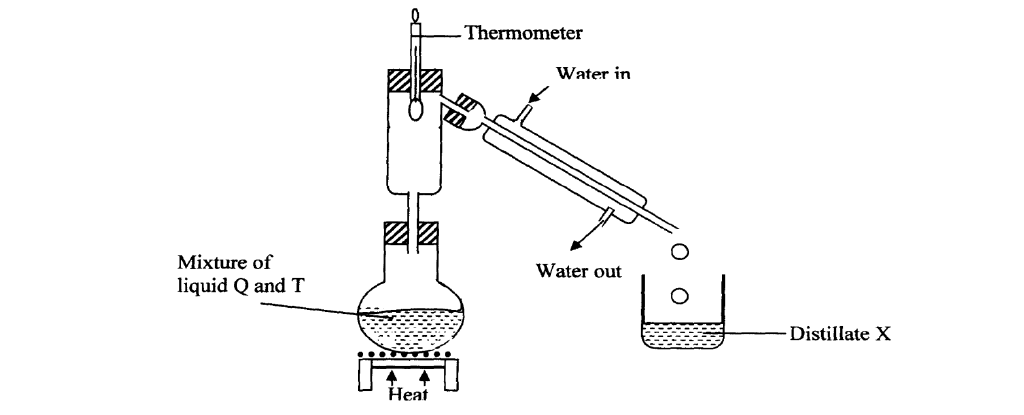
(a) Identify the mistakes in the setup above
(b)Identify Distillate X
Date posted:
October 11, 2019
.
Answers (1)
-
Given a mixture of lead (II) oxide, ammonium chloride and sodium chloride, describe how this mixture can be separated to obtain a sample of each.
(Solved)
Given a mixture of lead (II) oxide, ammonium chloride and sodium chloride, describe how this mixture can be separated to obtain a sample of each.
Date posted:
October 11, 2019
.
Answers (1)
-
Study the information below and answer the questions that follow:
(Solved)
Study the information below and answer the questions that follow:

Describe how the mixture of solid R, S, and V can be separated
Date posted:
October 11, 2019
.
Answers (1)
-
The set-up below was used to separate a mixture:-
(Solved)
The set-up below was used to separate a mixture:-
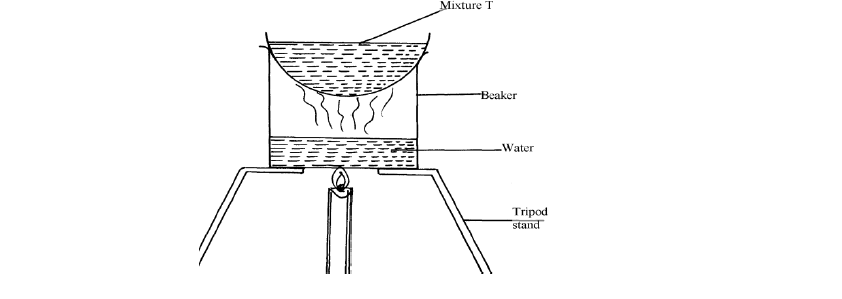
(a) Name the apparatus missing in the set-up
(b) Give one example of mixture T
(c) What is the name of this method of separation
Date posted:
October 11, 2019
.
Answers (1)
-
A student left some crushed fruit mixture with water for some days. He found the mixture had fermented. He concluded that the mixture was contaminated...
(Solved)
A student left some crushed fruit mixture with water for some days. He found the mixture had fermented. He concluded that the mixture was contaminated with water and ethanol with boiling point of 100oC and 78oC respectively. The set-up of apparatus below are used to separate the mixture.
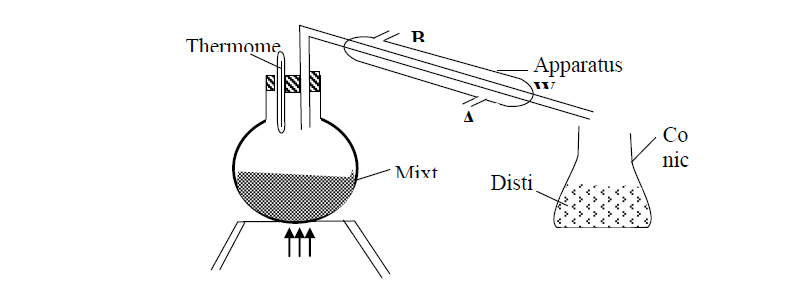
(i) Name the piece of apparatus labelled W
(ii) What is the purpose of the thermometer in the set-up?
iii) At which end of the apparatus W should tap water be connected?
(iv) Which liquid was collected as the first distillate? Explain
(v) What is the name given to the above method of separating mixture?
(vi) State two applications of the above method of separating mixtures
(vii) What properties of the mixture makes it possible for the component to be separated by the above methods?
Date posted:
October 11, 2019
.
Answers (1)
-
State two criteria for determining the purity of a substance
(Solved)
State two criteria for determining the purity of a substance
Date posted:
October 11, 2019
.
Answers (1)
-
The apparatus below were used by a student to study the effect of heat on hydrated copper II sulphate
(Solved)
The apparatus below were used by a student to study the effect of heat on hydrated copper II sulphate
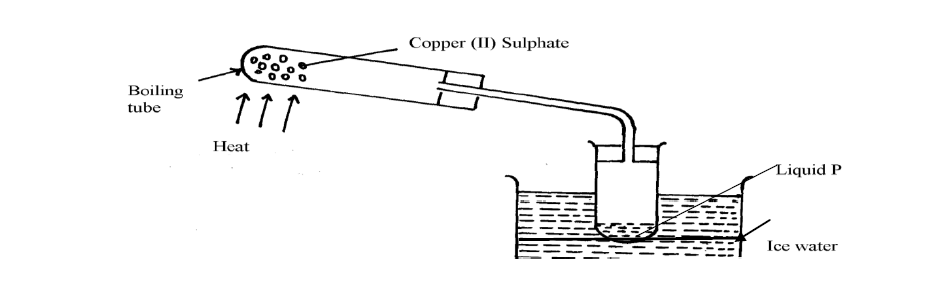
(a) What is the role of the ice cold water
(b) Name liquid P
(c) What observation is made in the boiling tube
Date posted:
October 11, 2019
.
Answers (1)
-
A form 1 student carried out the separation as shown in the set-up below:-
(Solved)
A form 1 student carried out the separation as shown in the set-up below:-
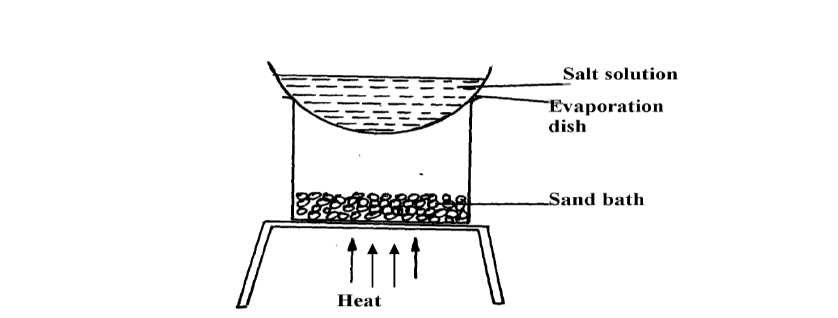
(i) Identify the method above.
(ii) Give one of its disadvantages
(iii) Name a mixture which can be separated by the set-up above
Date posted:
October 11, 2019
.
Answers (1)
-
Cooking oils comprise of a mixture of compounds which have a boiling point range
of 23oC to 27oC.
(i) What evidence is then to support the statement...
(Solved)
Cooking oils comprise of a mixture of compounds which have a boiling point range
of 23oC to 27oC.
(i) What evidence is there to support the statement that cooking oil is a mixture?
(ii)Name another experimental technique that could be used to confirm your answer in part (i) above
Date posted:
October 11, 2019
.
Answers (1)