(i) White fumes form in the gas jar which disappear after sometime.
- The level of water rises in the gas jar.
(ii) P(s) + O2(g) ------>P2O5(s)
P2 O(s) + 3H2O(l)--------->2H4PO4(aq)
(iii) Magnesium react with oxygen and nitrogen hence greater of fraction of air is used.
(iv) (a) Blue litmus changed to red as remained red. The solution was acid due to phosphoric
(b) Red litmus changed to blue as blue remained blue due to formation of basic magnesium hydroxide ammonia solution.
(v) – Pass air over conc. KOH / NaOH to absorb CO2
- Pass the remaining gases over hot copper solid which reacts with oxygen.
- Collect the remaining gas over water. The gas is mainly nitrogen.
maurice.mutuku answered the question on October 14, 2019 at 06:21
- An hydrocarbon can be represented as: C2 H2(Solved)
An hydrocarbon can be represented as: C2 H2
[a] Name the hydrocarbon.
[b] State two reagents that can be reacted together to generate the hydrocarbon.
[c] Identify the group of hydrocarbons into which C2 H2 belongs to.
Date posted: October 14, 2019. Answers (1)
- The reduction potentials of elements M and N are:(Solved)
The reduction potentials of elements M and N are:
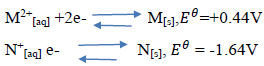
Using the above reduction potentials, predict whether a reaction would occur between
N+[aq] and M[[s]
Date posted: October 14, 2019. Answers (1)
- Draw a labelled set-up of apparatus for the laboratory preparation of oxygen using Sodium Peroxide(Solved)
Draw a labelled set-up of apparatus for the laboratory preparation of oxygen using Sodium Peroxide
Date posted: October 14, 2019. Answers (1)
- The table shows the colours obtained when some indicators are added to solutions:-(Solved)
The table shows the colours obtained when some indicators are added to solutions:-

(a) Complete the table by filling in the missing colours
(b) Identify indicator W
Date posted: October 14, 2019. Answers (1)
- In an experiment, ammonia gas was prepared by heating ammonium salt with an alkali.
After drying, ammonia gas was collected at room temperature and pressure.
(a) What...(Solved)
In an experiment, ammonia gas was prepared by heating ammonium salt with an alkali.
After drying, ammonia gas was collected at room temperature and pressure.
(a) What is meant by the term alkali?
(b) Explain using physical properties of the gas why ammonia is not collected by downward delivery
Date posted: October 14, 2019. Answers (1)
- The following data gives the pH values of some solutions(Solved)
The following data gives the pH values of some solutions

(a) What colour change would occur in solution P on addition of two drops of phenolphthalein indicator?
(b) State the pH value of a resulting solution when equal moles of solution P and R react
Date posted: October 14, 2019. Answers (1)
- Explain why very little Carbon (IV) oxide gas is evolved when dilute sulphuric (VI) acid is added to lead (II) carbonate(Solved)
Explain why very little Carbon (IV) oxide gas is evolved when dilute sulphuric (VI) acid is added to lead (II) carbonate
Date posted: October 14, 2019. Answers (1)
- Use the information given below to answer the questions that follow:(Solved)
Use the information given below to answer the questions that follow:
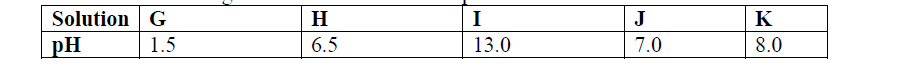
(a) Which of the solutions would be used to relieve a stomach upset caused by indigestion?
(b) Which solution is likely to be:
(i) Dilute sulphuric acid?
(ii) Sodium hydroxide solution?
Date posted: October 14, 2019. Answers (1)
- Complete the table below to show the colour of the given indicator in acidic and basic solutions.(Solved)
a) Complete the table below to show the colour of the given indicator in acidic and basic solutions.
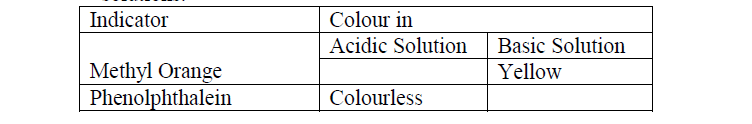
b) How does the PH value of 0.1M potassium hydroxide solution compare with that of 0.1M
aqueous ammonia? Explain.
Date posted: October 14, 2019. Answers (1)
- Below are the pH values of 4 types of medicine represented by letters P, Q, R and S(Solved)
Below are the pH values of 4 types of medicine represented by letters P, Q, R and S
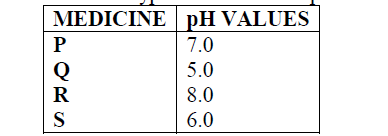
a) It is not advisable to use S when a patient has indigestion .Explain
b) What is the role of chemistry in drug manufacture
Date posted: October 11, 2019. Answers (1)
- a)Name two particles likely to be emitted when a radioactive nuclide undergoes radioactivity.
b)The half-life of a radioactive nuclide is 3 hours. Given that its initial...(Solved)
a)Name two particles likely to be emitted when a radioactive nuclide undergoes radioactivity.
b)The half-life of a radioactive nuclide is 3 hours. Given that its initial mass is 288g, determine the remaining mass after 12 hours.
Date posted: October 11, 2019. Answers (1)
- During the electrolysis of dilute copper {II} chloride using carbon electrodes, a current of 1.5A was passed through the solution for 2 hours and 30...(Solved)
During the electrolysis of dilute copper {II} chloride using carbon electrodes, a current of 1.5A was passed through the solution for 2 hours and 30 minutes.
[a] Write the ionic equation of the reaction that occurred at the cathode.
b] Given R.A.M of copper = 64 and 1F = 96500C, calculate the change in mass of the cathode.
Date posted: October 11, 2019. Answers (1)
- A reaction can be represented as;(Solved)
A reaction can be represented as;
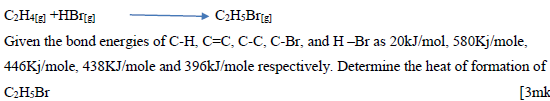
Date posted: October 11, 2019. Answers (1)
- A polymer can be represented as follows(Solved)
A polymer can be represented as follows

[a] Name and draw the structure of the monomer.
[b] What type of polymerization occurs in the above case?
[c] Given that the molecular mass of the polymer is 25620, how many units of the monomer make the polymer.
Date posted: October 11, 2019. Answers (1)
- At 600C, 38 grams of lead{II} nitrate saturate 56cm3 of water. Determine the solubility of lead {II} nitrate at this temperature.(Solved)
At 600C, 38 grams of lead{II} nitrate saturate 56cm3 of water. Determine the solubility of lead {II} nitrate at this temperature.
Date posted: October 11, 2019. Answers (1)
- Rusting is a process that causes massive destruction of iron structures
[a] State one condition that accelerates rusting.
[b] State one advantage of rusting(Solved)
Rusting is a process that causes massive destruction of iron structures.
[a] State one condition that accelerates rusting.
[b] State one advantage of rusting.
Date posted: October 11, 2019. Answers (1)
- Solid copper (II) oxide is a base although it does not turn litmus paper to blue. Explain(Solved)
Solid copper (II) oxide is a base although it does not turn litmus paper to blue. Explain
Date posted: October 11, 2019. Answers (1)
- When sodium nitrate was heated a solid A and gas B were produced identify solid A and gas B.(Solved)
When sodium nitrate was heated a solid A and gas B were produced identify solid A and gas B.
Date posted: October 11, 2019. Answers (1)
- Give one example of an acid salt.(Solved)
Give one example of an acid salt .
Date posted: October 11, 2019. Answers (1)
- The reduction potential of elements K, L, M, and P are as given below.(Solved)
The reduction potential of elements K, L, M, and P are as given below.
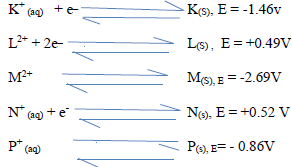
(i) Which letter represents the, strongest reducing agent? give a reason.
(ii) Which two letters represent elements whose half cells would form an electro chemical cell with the largest e.m.f?.
(iii) Calculate the e.m.f of the cell formed in (ii) above.
(d) During the electrolysis of a molten chloride of metal Q, a current of 0.25A was passed though the molten chloride for 2 hours and 10minutes. Given that 0.9grams of metal Q were deposited at the cathode.
(i) Calculate the quantity of electricity passed.
(ii) Charge carried by the ions of metal Q given that R.A.M of metal Q is 84.
Date posted: October 11, 2019. Answers (1)