-
(a) A group of students burnt a piece of Mg ribbon in air and its ash collected in a Petri dish.
The ash was found to...
(Solved)
(a) A group of students burnt a piece of Mg ribbon in air and its ash collected in a Petri dish.
The ash was found to comprise of magnesium Oxide and Magnesium nitride
(i) Write an equation for the reaction leading to formation of the magnesium nitride
(ii) A little water was added to the products in the Petri dish. State and explain the observation made.
(iii) A piece of blue litmus paper was dipped into the solution formed in (b) above.
State the observation made.
Date posted:
October 14, 2019
.
Answers (1)
-
In an experiment to investigate a certain property of sulphur, Maina added few drops of conc HNO3 to sulphur in a test tube and warmed...
(Solved)
In an experiment to investigate a certain property of sulphur, Maina added few drops of conc HNO3 to sulphur in a test tube and warmed the mixture.
[i]State one observation made.
[ii]Write a chemical equation of the reaction that occurred.
Date posted:
October 14, 2019
.
Answers (1)
-
The set-up below was used to investigate properties of the components of air:
(Solved)
The set-up below was used to investigate properties of the components of air:
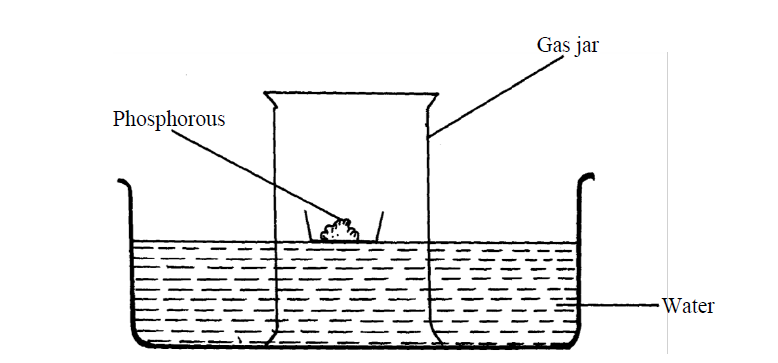
(i) State two observations made during the experiment
(ii) Write two chemical equations for the reactions which occurred
(iii) The experiment was repeated using burning magnesium in place of phosphorous.
There was greater rise of water than in the first case. Explain this observation
(iv) After the two experiments, the water in each trough was tested using blue and red litmus
papers. State and explain the observations of each case.
(a) Phosphorous experiment
b) magnesium experiment
(v) Briefly explain how a sample of nitrogen gas can be isolated from air in the laboratory
Date posted:
October 14, 2019
.
Answers (1)
-
Draw a labelled set-up of apparatus for the laboratory preparation of oxygen using Sodium Peroxide
(Solved)
Draw a labelled set-up of apparatus for the laboratory preparation of oxygen using Sodium Peroxide
Date posted:
October 14, 2019
.
Answers (1)
-
The table shows the colours obtained when some indicators are added to solutions:-
(Solved)
The table shows the colours obtained when some indicators are added to solutions:-

(a) Complete the table by filling in the missing colours
(b) Identify indicator W
Date posted:
October 14, 2019
.
Answers (1)
-
In an experiment, ammonia gas was prepared by heating ammonium salt with an alkali.
After drying, ammonia gas was collected at room temperature and pressure.
(a) What...
(Solved)
In an experiment, ammonia gas was prepared by heating ammonium salt with an alkali.
After drying, ammonia gas was collected at room temperature and pressure.
(a) What is meant by the term alkali?
(b) Explain using physical properties of the gas why ammonia is not collected by downward delivery
Date posted:
October 14, 2019
.
Answers (1)
-
The following data gives the pH values of some solutions
(Solved)
The following data gives the pH values of some solutions

(a) What colour change would occur in solution P on addition of two drops of phenolphthalein indicator?
(b) State the pH value of a resulting solution when equal moles of solution P and R react
Date posted:
October 14, 2019
.
Answers (1)
-
Explain why very little Carbon (IV) oxide gas is evolved when dilute sulphuric (VI) acid is added to lead (II) carbonate
(Solved)
Explain why very little Carbon (IV) oxide gas is evolved when dilute sulphuric (VI) acid is added to lead (II) carbonate
Date posted:
October 14, 2019
.
Answers (1)
-
Below are the pH values of 4 types of medicine represented by letters P, Q, R and S
(Solved)
Below are the pH values of 4 types of medicine represented by letters P, Q, R and S
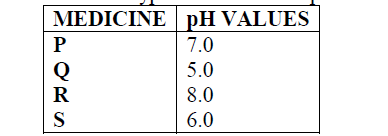
a) It is not advisable to use S when a patient has indigestion .Explain
b) What is the role of chemistry in drug manufacture
Date posted:
October 11, 2019
.
Answers (1)
-
During the electrolysis of dilute copper {II} chloride using carbon electrodes, a current of 1.5A was passed through the solution for 2 hours and 30...
(Solved)
During the electrolysis of dilute copper {II} chloride using carbon electrodes, a current of 1.5A was passed through the solution for 2 hours and 30 minutes.
[a] Write the ionic equation of the reaction that occurred at the cathode.
b] Given R.A.M of copper = 64 and 1F = 96500C, calculate the change in mass of the cathode.
Date posted:
October 11, 2019
.
Answers (1)
-
At 600C, 38 grams of lead{II} nitrate saturate 56cm3 of water. Determine the solubility of lead {II} nitrate at this temperature.
(Solved)
At 600C, 38 grams of lead{II} nitrate saturate 56cm3 of water. Determine the solubility of lead {II} nitrate at this temperature.
Date posted:
October 11, 2019
.
Answers (1)
-
Solid copper (II) oxide is a base although it does not turn litmus paper to blue. Explain
(Solved)
Solid copper (II) oxide is a base although it does not turn litmus paper to blue. Explain
Date posted:
October 11, 2019
.
Answers (1)
-
When sodium nitrate was heated a solid A and gas B were produced identify solid A and gas B.
(Solved)
When sodium nitrate was heated a solid A and gas B were produced identify solid A and gas B.
Date posted:
October 11, 2019
.
Answers (1)
-
The information below gives PH values of solutions V, W, X, Y Z
(Solved)
The information below gives PH values of solutions V, W, X, Y Z

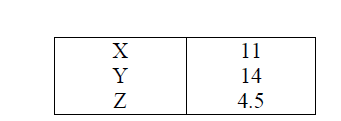
(a) Which solution is likely to be:
(i) Calcium hydroxide?
(ii) Rain water?
(b) Which solution would react most vigorously with Zinc carbonate
Date posted:
October 11, 2019
.
Answers (1)
-
The diagram below shows the contact process used in the manufacture of concentrated sulphuric(vi) acid.
(Solved)
The diagram below shows the contact process used in the manufacture of concentrated sulphuric(vi) acid.
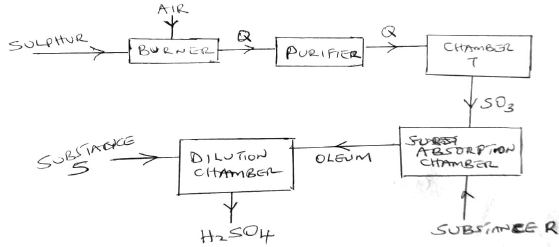
a) Identify the following:
i) Substance Q formed in the burner
ii) Chamber T
iii) Substance R
iv) Substance S
ii) Write the chemical equation occurring in the dilution chamber.
iii) Why is it necessary to pass substance Q though a purifier.
iv) State one use of sulphuric (VI) acid.
Date posted:
October 11, 2019
.
Answers (1)
-
The diagram below shows the process used to obtain Sulphur from underground deposits.
(Solved)
The diagram below shows the process used to obtain Sulphur from underground deposits.
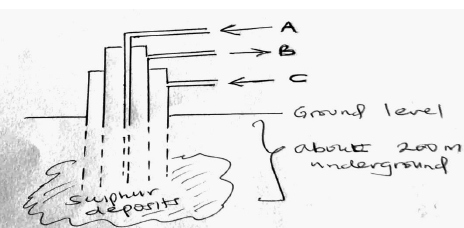
i) Name the above process used to obtain sulphur from the underground deposits
ii) Name the substance passed through pipe
A
B
iii) State two properties of Sulphur that makes it possible to extract using the above process.
Date posted:
October 11, 2019
.
Answers (1)
-
Name the following compounds
(i) CH3CH2CH2COOH
(Solved)
Name the following compounds
(i) CH3CH2CH2COOH
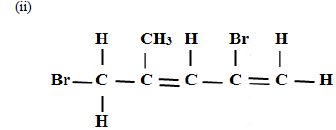
(iii)CH3CH2OOCCH2CH3
b) Two types of detergents P and Q can be represented as
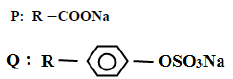
(i) Identify each type of the detergent.
(ii) Which of the two detergents is the best to use with hard water? Give a reason.
(iii) State one advantage of detergent P.
(iv) State one disadvantage of detergent Q.
(c) An hydrocarbon can be represented as follows.
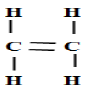
(i) Identify the hydrocarbon
(ii) Name two reagents that can reacted together to generate the hydrocarbon
Date posted:
October 11, 2019
.
Answers (1)
-
The setup below was used to separate two miscible liquids Q and T
(Boling points; Q =98° C, T=78°C)
(Solved)
The setup below was used to separate two miscible liquids Q and T
(Boling points; Q =98° C, T=78°C)
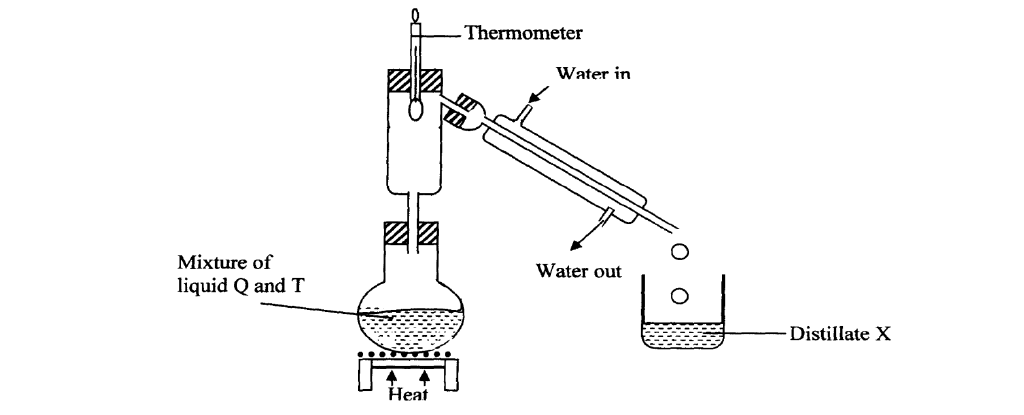
(a) Identify the mistakes in the setup above
(b)Identify Distillate X
Date posted:
October 11, 2019
.
Answers (1)
-
Given a mixture of lead (II) oxide, ammonium chloride and sodium chloride, describe how this mixture can be separated to obtain a sample of each.
(Solved)
Given a mixture of lead (II) oxide, ammonium chloride and sodium chloride, describe how this mixture can be separated to obtain a sample of each.
Date posted:
October 11, 2019
.
Answers (1)
-
Study the information below and answer the questions that follow:
(Solved)
Study the information below and answer the questions that follow:

Describe how the mixture of solid R, S, and V can be separated
Date posted:
October 11, 2019
.
Answers (1)