(i) The reaction is too exothermic that a lot of heat is produced causing ignition of hydrogen in presence of oxygen
(ii) K(s) + H2O(g)------->KOH(aq) + H2(g)
H2(g) + O2(g)----->H2O(g)
maurice.mutuku answered the question on October 14, 2019 at 07:25
- Hydrogen can reduce coppers Oxide but not aluminium oxide. Explain(Solved)
Hydrogen can reduce coppers Oxide but not aluminium oxide. Explain
Date posted: October 14, 2019. Answers (1)
- Fe3O4 and FeO are oxides of iron which can be produced in the laboratory
(a) Write chemical equation for the reaction which can be used to...(Solved)
Fe3O4 and FeO are oxides of iron which can be produced in the laboratory
(a) Write chemical equation for the reaction which can be used to produce each of the oxides
(b) Write an ionic equation for the reaction between the oxide, Fe3O4 and a dilute acid.
Date posted: October 14, 2019. Answers (1)
- A piece of phosphorous was burnt in excess air. The product obtained was shaken with a small amount of hot water to make a solution
i)...(Solved)
A piece of phosphorous was burnt in excess air. The product obtained was shaken with a small amount of hot water to make a solution
i) Write an equation for the burning of phosphorus in excess air
ii) The solution obtained above was found to have pH of 2. Give reasons for this observation
Date posted: October 14, 2019. Answers (1)
- The electron configuration of elements A, B, C, D and E are as given below.(Solved)
The electron configuration of elements A, B, C, D and E are as given below.

{a} Which element has the highest electrical conductivity.
{b} Which letter represents the most reactive metal.
c} Which letter represents the most reactive non-metal.
Date posted: October 14, 2019. Answers (1)
- Given mass of gas T diffuses through a porous plug in 48 seconds while a similar mass of gas R diffuse in 70 seconds. Given...(Solved)
Given mass of gas T diffuses through a porous plug in 48 seconds while a similar mass of gas R diffuse in 70 seconds. Given that the density of gas T is 0.6g/cm3, find the density of gas R.
Date posted: October 14, 2019. Answers (1)
- Element W has two isotopes W – 36 and W-40 which occur in the ratio x:4. Given that R.A.M of W is 37.25, find the...(Solved)
Element W has two isotopes W – 36 and W-40 which occur in the ratio x:4. Given that R.A.M of W is 37.25, find the value of x.
Date posted: October 14, 2019. Answers (1)
- Gas X and Y can be collected as shown below.(Solved)
Gas X and Y can be collected as shown below.

[a] Name the method used to collect gas Y.
[b] How do densities of gas X and gas Y compare?
[c]Give an example of a gas that can be collected using the same method as gas Y.
Date posted: October 14, 2019. Answers (1)
- In the industrial manufacture of Ammonia one of the raw materials is nitrogen gas
{a} Name one other raw material(Solved)
In the industrial manufacture of Ammonia one of the raw materials is nitrogen gas
{a} Name one other raw material
{b} Name two possible sources of the raw material you have named in {a} above
{c} Name two substances that can be used as catalyst in this process
{d} State one use of ammonia
Date posted: October 14, 2019. Answers (1)
- Few drops of hydrochloric acid were added into a test tube containing lead {II} Nitrate solution.(Solved)
Few drops of hydrochloric acid were added into a test tube containing lead {II} Nitrate solution.
{a} State one observation made
{b} Write an ionic equation of the reaction that occurred in the test tube
Date posted: October 14, 2019. Answers (1)
- When 20g of a compound containing carbon, hydrogen and oxygen was burnt in the air, 29.3g of carbon{IV} oxide and 11.7g of water were produced....(Solved)
When 20g of a compound containing carbon, hydrogen and oxygen was burnt in the air, 29.3g of carbon{IV} oxide and 11.7g of water were produced. Determine its empirical formulae.
{C=12, H=1 , O=16}
Date posted: October 14, 2019. Answers (1)
- (a) A group of students burnt a piece of Mg ribbon in air and its ash collected in a Petri dish.
The ash was found to...(Solved)
(a) A group of students burnt a piece of Mg ribbon in air and its ash collected in a Petri dish.
The ash was found to comprise of magnesium Oxide and Magnesium nitride
(i) Write an equation for the reaction leading to formation of the magnesium nitride
(ii) A little water was added to the products in the Petri dish. State and explain the observation made.
(iii) A piece of blue litmus paper was dipped into the solution formed in (b) above.
State the observation made.
Date posted: October 14, 2019. Answers (1)
- A compound can be represented as(Solved)
A compound can be represented as
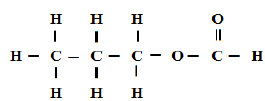
[a] What name is given to the above class of compounds.
[b] Name two reagents that can be reacted together to generate the above compound.
[c] State two conditions necessary for the reaction leading to formation of the above compound to occur.
Date posted: October 14, 2019. Answers (1)
- Chlorine is commonly used in the manufacture of Ca (OCl)2
[i] State one use of the above compound of chlorine
ii] Write a chemical equation leading to...(Solved)
Chlorine is commonly used in the manufacture of Ca (OCl)2
[i] State one use of the above compound of chlorine
ii] Write a chemical equation leading to the production of Ca (OCl)2
Date posted: October 14, 2019. Answers (1)
- In an experiment to investigate a certain property of sulphur, Maina added few drops of conc HNO3 to sulphur in a test tube and warmed...(Solved)
In an experiment to investigate a certain property of sulphur, Maina added few drops of conc HNO3 to sulphur in a test tube and warmed the mixture.
[i]State one observation made.
[ii]Write a chemical equation of the reaction that occurred.
Date posted: October 14, 2019. Answers (1)
- The set-up below was used to investigate properties of the components of air:(Solved)
The set-up below was used to investigate properties of the components of air:
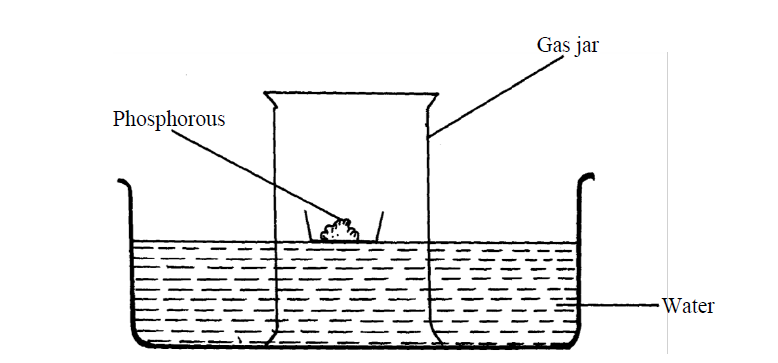
(i) State two observations made during the experiment
(ii) Write two chemical equations for the reactions which occurred
(iii) The experiment was repeated using burning magnesium in place of phosphorous.
There was greater rise of water than in the first case. Explain this observation
(iv) After the two experiments, the water in each trough was tested using blue and red litmus
papers. State and explain the observations of each case.
(a) Phosphorous experiment
b) magnesium experiment
(v) Briefly explain how a sample of nitrogen gas can be isolated from air in the laboratory
Date posted: October 14, 2019. Answers (1)
- An hydrocarbon can be represented as: C2 H2(Solved)
An hydrocarbon can be represented as: C2 H2
[a] Name the hydrocarbon.
[b] State two reagents that can be reacted together to generate the hydrocarbon.
[c] Identify the group of hydrocarbons into which C2 H2 belongs to.
Date posted: October 14, 2019. Answers (1)
- The reduction potentials of elements M and N are:(Solved)
The reduction potentials of elements M and N are:
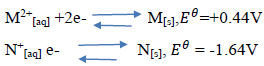
Using the above reduction potentials, predict whether a reaction would occur between
N+[aq] and M[[s]
Date posted: October 14, 2019. Answers (1)
- Draw a labelled set-up of apparatus for the laboratory preparation of oxygen using Sodium Peroxide(Solved)
Draw a labelled set-up of apparatus for the laboratory preparation of oxygen using Sodium Peroxide
Date posted: October 14, 2019. Answers (1)
- The table shows the colours obtained when some indicators are added to solutions:-(Solved)
The table shows the colours obtained when some indicators are added to solutions:-

(a) Complete the table by filling in the missing colours
(b) Identify indicator W
Date posted: October 14, 2019. Answers (1)
- In an experiment, ammonia gas was prepared by heating ammonium salt with an alkali.
After drying, ammonia gas was collected at room temperature and pressure.
(a) What...(Solved)
In an experiment, ammonia gas was prepared by heating ammonium salt with an alkali.
After drying, ammonia gas was collected at room temperature and pressure.
(a) What is meant by the term alkali?
(b) Explain using physical properties of the gas why ammonia is not collected by downward delivery
Date posted: October 14, 2019. Answers (1)