- 10.08g of ethanedioic acid (H2C2O4.xH2O) crystals were dissolved in water and made to 1dm3 solution. 25.0cm3 of this solution was completely neutralized by 20cm3 of...(Solved)
10.08g of ethanedioic acid (H2C2O4.xH2O) crystals were dissolved in water and made to 1dm3 solution. 25.0cm3 of this solution was completely neutralized by 20cm3 of 0.2M
sodium hydroxide solution.
Calculate
i) Molarity of the acid
ii)the value of x in H2C2O4xH2O acid
Date posted: October 17, 2019. Answers (1)
- Campers GAZ cylinder contains about 1.12dm3 of butane measured at 0o and 1atm. Given that 25% of heat is lost, what is the maximum volume...(Solved)
Campers GAZ cylinder contains about 1.12dm3 of butane measured at 0o and 1atm. Given that 25% of heat is lost, what is the maximum volume of water at room temperature which can be boiled to 100oC in order to make some coffee?
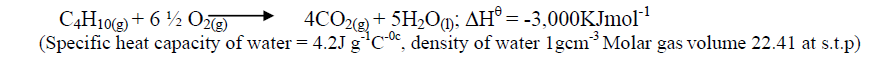
Date posted: October 17, 2019. Answers (1)
- An organic compound P contains 68.9% carbon, 13.5% hydrogen and 21.6% oxygen.
The relative formula mass of p is 74. Determine its molecular formula. [C=12, H=1,...(Solved)
An organic compound P contains 68.9% carbon, 13.5% hydrogen and 21.6% oxygen.
The relative formula mass of p is 74. Determine its molecular formula. [C=12, H=1, 0=16]
Date posted: October 17, 2019. Answers (1)
- In a reaction 20cm3 of 0.1 M Sodium Carbonate completely reacted with 13cm3 of dilute sulphuric acid. Find the molarity of the sulphuric acid used.(Solved)
In a reaction 20cm3 of 0.1 M Sodium Carbonate completely reacted with 13cm3 of dilute sulphuric acid. Find the molarity of the sulphuric acid used.
Date posted: October 17, 2019. Answers (1)
- Calculate the number of water molecules when 34.8g Na2CO3 xH2O is heated and 15.9g of anhydrous Na2CO3 obtained (H=1, O=16, Na= 23, C = 12)(Solved)
Calculate the number of water molecules when 34.8g Na2CO3 xH2O is heated and 15.9g of anhydrous Na2CO3 obtained (H=1, O=16, Na= 23, C = 12)
Date posted: October 17, 2019. Answers (1)
- 3.52g of Carbon (IV) Oxide and 1.40g of water are produced when a mass of a hydrocarbon is completely burnt in oxygen. Determine the empirical...(Solved)
3.52g of Carbon (IV) Oxide and 1.40g of water are produced when a mass of a hydrocarbon is completely burnt in oxygen. Determine the empirical formula of the hydrocarbon;
(H = 1 , C= 12, O = 16)
Date posted: October 17, 2019. Answers (1)
- Galvanized iron sheets are made by dipping the sheets in molten Zinc.
i) Explain how zinc protects iron from rusting
ii) Name the process applied in galvanization...(Solved)
Galvanized iron sheets are made by dipping the sheets in molten Zinc.
i) Explain how zinc protects iron from rusting
ii) Name the process applied in galvanization of iron with zinc
Date posted: October 17, 2019. Answers (1)
- Calculate the volume of oxygen gas used during the burning of magnesium (O = 16, molar gas volume = 24,000cm3 at room temperature)(Solved)
Calculate the volume of oxygen gas used during the burning of magnesium (O = 16, molar gas volume = 24,000cm3 at room temperature)
Date posted: October 17, 2019. Answers (1)
- How many chloride ions are present in 1.7g of magnesium chloride crystals?
(Avogadro’s constant = 6.0 x 1023, Mg = 24, Cl = 35.5)(Solved)
How many chloride ions are present in 1.7g of magnesium chloride crystals?
(Avogadro’s constant = 6.0 x 1023, Mg = 24, Cl = 35.5)
Date posted: October 17, 2019. Answers (1)
- When 1.675g of hydrated sodium carbonate was reacted with excess hydrochloric acid,
the volume carbon (IV) oxide gas obtained at room temperature and pressure was 150cm3).
Calculate...(Solved)
When 1.675g of hydrated sodium carbonate was reacted with excess hydrochloric acid,
the volume carbon (IV) oxide gas obtained at room temperature and pressure was 150cm3).
Calculate the number of moles of water of crystallization in one mole of hydrated sodium
carbonate:- (Na=23, H =1, C=12, O=16, MGV at R.T.P = 24000cm3)
Date posted: October 17, 2019. Answers (1)
- The equation of the formation of iron (III) chloride is
2Fe(s) + 3Cl2(g)------>2FeCl3
Calculate the volume of chlorine which will react with iron to form 0.5g of...(Solved)
The equation of the formation of iron (III) chloride is
2Fe(s) + 3Cl2(g)------>2FeCl3
Calculate the volume of chlorine which will react with iron to form 0.5g of Iron (III) chloride.
(Fe = 56 Cl=35.5). Molar gas volume at 298K = 24dm3)
Date posted: October 17, 2019. Answers (1)
- In a filtration experiment 25cm3 of a solution of Sodium Hydroxide containing 8g per
litre was required for complete neutralization of 0.245g of a dibasic acid....(Solved)
In a filtration experiment 25cm3 of a solution of Sodium Hydroxide containing 8g per
litre was required for complete neutralization of 0.245g of a dibasic acid. Calculate
the relative molecular mass of the acid (Na = 23.0, O = 16, H= 1)
Date posted: October 17, 2019. Answers (1)
- In an experiment magnesium ribbon was heated in air. The product formed was found to be heavier than the original ribbon. Potassium manganate (VII) was...(Solved)
In an experiment magnesium ribbon was heated in air. The product formed was found to be heavier than the original ribbon. Potassium manganate (VII) was on the other hand, heated in air and product formed was found to be lighter. Explain the differences on the observation made
Date posted: October 17, 2019. Answers (1)
- A fixed mass of gas occupies 200 cm3 at a temperature of 230c and a pressure of 740 mm Hg.
Calculate the volume of the gas...(Solved)
A fixed mass of gas occupies 200 cm3 at a temperature of 230c and a pressure of 740 mm Hg.
Calculate the volume of the gas at -250c and 790 mm Hg pressure.
Date posted: October 17, 2019. Answers (1)
- A gas occupies 5dm3 at a temperature of -270C and 1 atmosphere pressure. Calculate the volume occupied by the gas at a pressure of 2...(Solved)
A gas occupies 5dm3 at a temperature of -270C and 1 atmosphere pressure. Calculate the volume occupied by the gas at a pressure of 2 atmospheres and a temperature of 1270C
Date posted: October 17, 2019. Answers (1)
- The figure below shows two gases P and Q diffusing from two opposite ends 18 seconds after the experiment(Solved)
The figure below shows two gases P and Q diffusing from two opposite ends 18 seconds after the experiment

(a) Which of the gases has a lighter density?
(b) Given that the molecular mass of gas Q is 17, calculate the molecular mass of P
Date posted: October 17, 2019. Answers (1)
- Gas B takes 110 seconds to diffuse through a porous pot, how long will it take for the same amount of ammonia to diffuse under...(Solved)
Gas B takes 110 seconds to diffuse through a porous pot, how long will it take for the same amount of ammonia to diffuse under the same conditions of temperature and pressure?
(RMM of B = 34 RMM of ammonia = 17)
Date posted: October 17, 2019. Answers (1)
- Below are structures of particles. Use it to answer questions that follow. In each case only electrons in the outermost energy level are shown(Solved)
Below are structures of particles. Use it to answer questions that follow. In each case only electrons in the outermost energy level are shown

(a) Identify the particle which is an anion
(b) Choose a pair of isotopes. Give a reason
Date posted: October 17, 2019. Answers (1)
- A sample of Carbon (IV) Oxide takes 200 seconds to diffuse across a porous plug.
How long will it take the same amount of Carbon (II)...(Solved)
A sample of Carbon (IV) Oxide takes 200 seconds to diffuse across a porous plug.
How long will it take the same amount of Carbon (II) Oxide to diffuse through the same plug?(C=12, O=16)
Date posted: October 17, 2019. Answers (1)
- A fixed mass of gas occupies 200cm3 at a temperature of 23oC and pressure of 740mmHg.
Calculate the volume of the gas at -25oC and 780mmHg...(Solved)
A fixed mass of gas occupies 200cm3 at a temperature of 23oC and pressure of 740mmHg.
Calculate the volume of the gas at -25oC and 780mmHg pressure
Date posted: October 17, 2019. Answers (1)