(i) Gas /vapour
(ii) B - It has the second lowest boiling point thus second lowest molecular mass
(iii) C is impure since it boils over a range of temperature
(iv) It is boiled heated and the vapour of the components condense at different temperatures
(v) - Liquid air
- Crude oil
maurice.mutuku answered the question on October 18, 2019 at 05:18
- Give the open structures of:-
(i) 3-chlorohex-l-yne
(ii) CH3OH(Solved)
Give the open structures of:-
(i) 3-chlorohex-l-yne
(ii) CH3OH
Date posted: October 18, 2019. Answers (1)
- 10cm3 of methane (CH4) gas is exploded with 150cm3 of air containing 20% oxygen and 80% nitrogen. The products were allowed to cool to room...(Solved)
10cm3 of methane (CH4) gas is exploded with 150cm3 of air containing 20% oxygen and 80% nitrogen. The products were allowed to cool to room temperature. What will be the total volume of the gases at the end of the reaction?
Date posted: October 17, 2019. Answers (1)
- Polymerisation of ethene takes place as shown in the equation below(Solved)
Polymerisation of ethene takes place as shown in the equation below:
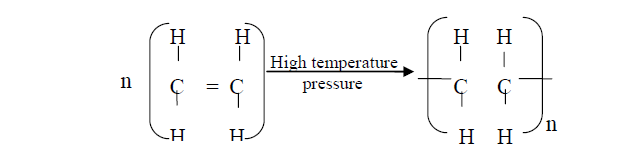
Name the type of polymerisation undergone by ethene in the reaction above
Date posted: October 17, 2019. Answers (1)
- Distinguish between the isotopes and isomers(Solved)
Distinguish between the isotopes and isomers
Date posted: October 17, 2019. Answers (1)
- In a reaction an alcohol K was converted to hex-1-ene
Name reagent and condition necessary for the reaction above to occur(Solved)
In a reaction an alcohol K was converted to hex-1-ene
Name reagent and condition necessary for the reaction above to occur
Date posted: October 17, 2019. Answers (1)
- In petrol chemical industries, long chain alkanes are broken down in to simpler substances in a process called cracking
a) Why is cracking necessary?
b) State the...(Solved)
In petrol chemical industries, long chain alkanes are broken down in to simpler substances in a process called cracking
a) Why is cracking necessary?
b) State the two conditions required in cracking
c) Draw the structure of 1-chloro-2, 2-dimethylpropane
Date posted: October 17, 2019. Answers (1)
- But-z-ene undergoes hydrogenation according to the equation given below(Solved)
But-z-ene undergoes hydrogenation according to the equation given below
CH3CH = CHCH3(g) + H2(g)----->CH3CH2CH2CH3(g)
Name the product formed when but-z-ene reacts with hydrogen gas
Date posted: October 17, 2019. Answers (1)
- Use the flow chart below to answer the questions that follow:(Solved)
Use the flow chart below to answer the questions that follow:
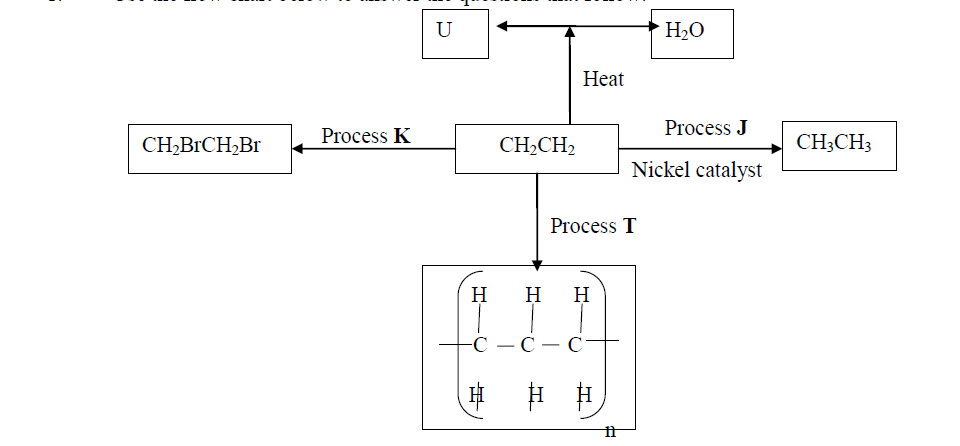
(a) What observation would be made in process K?
(b) Name another conditions necessary for process J to take place
(c) Give the name of substance V
Date posted: October 17, 2019. Answers (1)
- During welding of cracked railway lines by thermite 12.0g of oxide of iron is reduced by aluminium to 8.40g of iron. Determine the empirical formula...(Solved)
During welding of cracked railway lines by thermite 12.0g of oxide of iron is reduced by aluminium to 8.40g of iron. Determine the empirical formula of the oxide(Fe= 56.0, O= 16.0)
Date posted: October 17, 2019. Answers (1)
- 1.6g of magnesium metal is reacted with excess hydrochloric acid. Calculate the volume
of hydrogen gas produced (Molar gas volume at stp = 22.4dm3 Mg=24)(Solved)
1.6g of magnesium metal is reacted with excess hydrochloric acid. Calculate the volume
of hydrogen gas produced (Molar gas volume at stp = 22.4dm3 Mg=24)
Date posted: October 17, 2019. Answers (1)
- 10.08g of ethanedioic acid (H2C2O4.xH2O) crystals were dissolved in water and made to 1dm3 solution. 25.0cm3 of this solution was completely neutralized by 20cm3 of...(Solved)
10.08g of ethanedioic acid (H2C2O4.xH2O) crystals were dissolved in water and made to 1dm3 solution. 25.0cm3 of this solution was completely neutralized by 20cm3 of 0.2M
sodium hydroxide solution.
Calculate
i) Molarity of the acid
ii)the value of x in H2C2O4xH2O acid
Date posted: October 17, 2019. Answers (1)
- Campers GAZ cylinder contains about 1.12dm3 of butane measured at 0o and 1atm. Given that 25% of heat is lost, what is the maximum volume...(Solved)
Campers GAZ cylinder contains about 1.12dm3 of butane measured at 0o and 1atm. Given that 25% of heat is lost, what is the maximum volume of water at room temperature which can be boiled to 100oC in order to make some coffee?
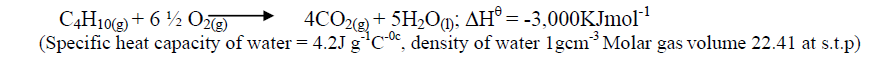
Date posted: October 17, 2019. Answers (1)
- An organic compound P contains 68.9% carbon, 13.5% hydrogen and 21.6% oxygen.
The relative formula mass of p is 74. Determine its molecular formula. [C=12, H=1,...(Solved)
An organic compound P contains 68.9% carbon, 13.5% hydrogen and 21.6% oxygen.
The relative formula mass of p is 74. Determine its molecular formula. [C=12, H=1, 0=16]
Date posted: October 17, 2019. Answers (1)
- In a reaction 20cm3 of 0.1 M Sodium Carbonate completely reacted with 13cm3 of dilute sulphuric acid. Find the molarity of the sulphuric acid used.(Solved)
In a reaction 20cm3 of 0.1 M Sodium Carbonate completely reacted with 13cm3 of dilute sulphuric acid. Find the molarity of the sulphuric acid used.
Date posted: October 17, 2019. Answers (1)
- Calculate the number of water molecules when 34.8g Na2CO3 xH2O is heated and 15.9g of anhydrous Na2CO3 obtained (H=1, O=16, Na= 23, C = 12)(Solved)
Calculate the number of water molecules when 34.8g Na2CO3 xH2O is heated and 15.9g of anhydrous Na2CO3 obtained (H=1, O=16, Na= 23, C = 12)
Date posted: October 17, 2019. Answers (1)
- 3.52g of Carbon (IV) Oxide and 1.40g of water are produced when a mass of a hydrocarbon is completely burnt in oxygen. Determine the empirical...(Solved)
3.52g of Carbon (IV) Oxide and 1.40g of water are produced when a mass of a hydrocarbon is completely burnt in oxygen. Determine the empirical formula of the hydrocarbon;
(H = 1 , C= 12, O = 16)
Date posted: October 17, 2019. Answers (1)
- Galvanized iron sheets are made by dipping the sheets in molten Zinc.
i) Explain how zinc protects iron from rusting
ii) Name the process applied in galvanization...(Solved)
Galvanized iron sheets are made by dipping the sheets in molten Zinc.
i) Explain how zinc protects iron from rusting
ii) Name the process applied in galvanization of iron with zinc
Date posted: October 17, 2019. Answers (1)
- Calculate the volume of oxygen gas used during the burning of magnesium (O = 16, molar gas volume = 24,000cm3 at room temperature)(Solved)
Calculate the volume of oxygen gas used during the burning of magnesium (O = 16, molar gas volume = 24,000cm3 at room temperature)
Date posted: October 17, 2019. Answers (1)
- How many chloride ions are present in 1.7g of magnesium chloride crystals?
(Avogadro’s constant = 6.0 x 1023, Mg = 24, Cl = 35.5)(Solved)
How many chloride ions are present in 1.7g of magnesium chloride crystals?
(Avogadro’s constant = 6.0 x 1023, Mg = 24, Cl = 35.5)
Date posted: October 17, 2019. Answers (1)
- When 1.675g of hydrated sodium carbonate was reacted with excess hydrochloric acid,
the volume carbon (IV) oxide gas obtained at room temperature and pressure was 150cm3).
Calculate...(Solved)
When 1.675g of hydrated sodium carbonate was reacted with excess hydrochloric acid,
the volume carbon (IV) oxide gas obtained at room temperature and pressure was 150cm3).
Calculate the number of moles of water of crystallization in one mole of hydrated sodium
carbonate:- (Na=23, H =1, C=12, O=16, MGV at R.T.P = 24000cm3)
Date posted: October 17, 2019. Answers (1)