i) Polythene
ii) Bubble pass ethane gas in acidified KMnO4 or acidified K2Cr2OT
maurice.mutuku answered the question on October 22, 2019 at 06:04
- Give the names of the following:
(i) CH3CH2CH3
(ii) CH3CCCH3(Solved)
Give the names of the following:
(i) CH3CH2CH3
(ii) CH3CCCH3
Date posted: October 22, 2019. Answers (1)
- Name the following compounds:(CH3)3 C CH2 CH2 CH3(Solved)
Name the following compounds:(CH3)3 C CH2 CH2 CH3
Date posted: October 22, 2019. Answers (1)
- Give two ways how the disposal of polymers such as polychloroethene by burning pollutes the environment(Solved)
Give two ways how the disposal of polymers such as polychloroethene by burning pollutes the environment
Date posted: October 22, 2019. Answers (1)
- State the observations made when buton-l-ol reacts with:-
(i) Acidified potassium dichromate (VI) solution
(ii) Potassium metal(Solved)
State the observations made when buton-l-ol reacts with:-
(i) Acidified potassium dichromate (VI) solution
(ii) Potassium metal
Date posted: October 22, 2019. Answers (1)
- Give the systematic names of the following compounds:-(Solved)
Give the systematic names of the following compounds:-

Date posted: October 22, 2019. Answers (1)
- Use the diagram below to answer the questions that follow:(Solved)
Use the diagram below to answer the questions that follow:
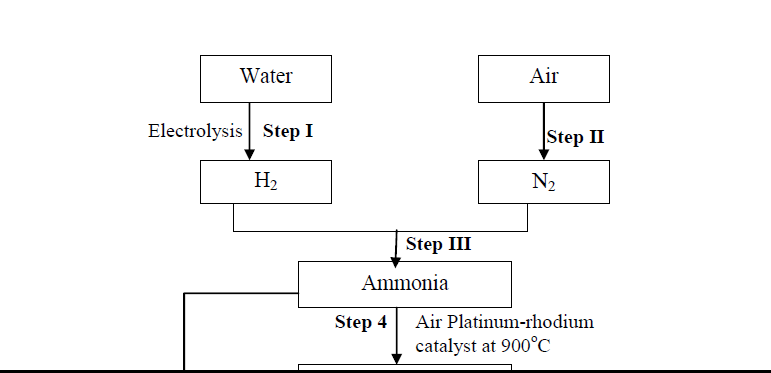
(i) Name another source of hydrogen apart from electrolysis of water
(ii) What conditions are necessary for step III to occur?
(iii) Write the equation for the formation of colourless gas Q
Date posted: October 22, 2019. Answers (1)
- Given the reaction:(Solved)
Given the reaction:

(i) Identify substance and F and N
(ii) Name the process represented above?
(iii) Give one use of substance N
Date posted: October 18, 2019. Answers (1)
- (i) Complete the equation below :
CH3COOCH3 + H2O
(ii) What type of reaction is occurring above(Solved)
(i) Complete the equation below :
CH3COOCH3 + H2O
(ii) What type of reaction is occurring above
Date posted: October 18, 2019. Answers (1)
- The set-up below was used to prepare ethyne gas(Solved)
The set-up below was used to prepare ethyne gas
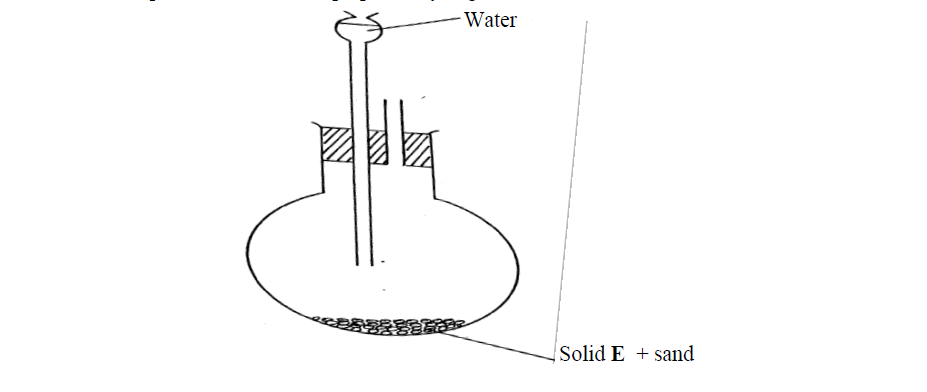
(i) Identify solid E
(ii) Complete the diagram to show how the gas can be collected
(iii) Write an equation to show how the gas is formed
(iv) Complete the equation below: )
C2H2 + 2I2---------------->
(v) What is the role of sand in the experiment?
Date posted: October 18, 2019. Answers (1)
- The diagram below represents a large-scale fractional distillation plant used to separate the components A, B, C and D in a mixture(Solved)
The diagram below represents a large-scale fractional distillation plant used to separate the components A, B, C and D in a mixture
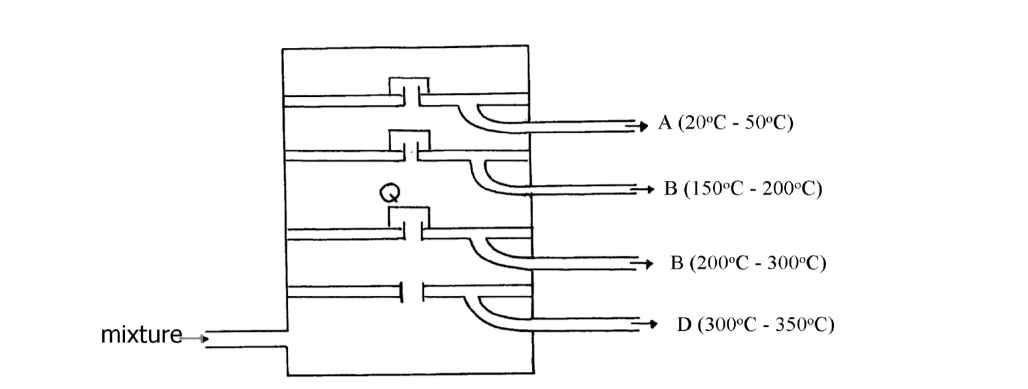
The components have the following average relative molecular masses not necessarily in that
order; 282, 184, 44 and 128.
(i) What is the physical state of B at the position marked Q?
(ii) Which component has an average relative molecular mass of 128? Explain
(iii) State with a reason whether C is pure or impure
(iv) Explain how the mixture is separated into its components
(v) Name two naturally occurring mixtures that are separated using this process
Date posted: October 18, 2019. Answers (1)
- Give the open structures of:-
(i) 3-chlorohex-l-yne
(ii) CH3OH(Solved)
Give the open structures of:-
(i) 3-chlorohex-l-yne
(ii) CH3OH
Date posted: October 18, 2019. Answers (1)
- 10cm3 of methane (CH4) gas is exploded with 150cm3 of air containing 20% oxygen and 80% nitrogen. The products were allowed to cool to room...(Solved)
10cm3 of methane (CH4) gas is exploded with 150cm3 of air containing 20% oxygen and 80% nitrogen. The products were allowed to cool to room temperature. What will be the total volume of the gases at the end of the reaction?
Date posted: October 17, 2019. Answers (1)
- Polymerisation of ethene takes place as shown in the equation below(Solved)
Polymerisation of ethene takes place as shown in the equation below:
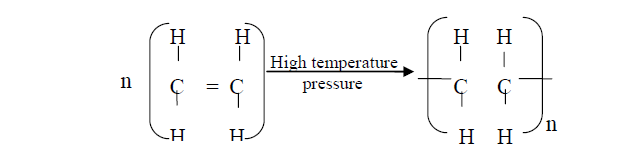
Name the type of polymerisation undergone by ethene in the reaction above
Date posted: October 17, 2019. Answers (1)
- Distinguish between the isotopes and isomers(Solved)
Distinguish between the isotopes and isomers
Date posted: October 17, 2019. Answers (1)
- In a reaction an alcohol K was converted to hex-1-ene
Name reagent and condition necessary for the reaction above to occur(Solved)
In a reaction an alcohol K was converted to hex-1-ene
Name reagent and condition necessary for the reaction above to occur
Date posted: October 17, 2019. Answers (1)
- In petrol chemical industries, long chain alkanes are broken down in to simpler substances in a process called cracking
a) Why is cracking necessary?
b) State the...(Solved)
In petrol chemical industries, long chain alkanes are broken down in to simpler substances in a process called cracking
a) Why is cracking necessary?
b) State the two conditions required in cracking
c) Draw the structure of 1-chloro-2, 2-dimethylpropane
Date posted: October 17, 2019. Answers (1)
- But-z-ene undergoes hydrogenation according to the equation given below(Solved)
But-z-ene undergoes hydrogenation according to the equation given below
CH3CH = CHCH3(g) + H2(g)----->CH3CH2CH2CH3(g)
Name the product formed when but-z-ene reacts with hydrogen gas
Date posted: October 17, 2019. Answers (1)
- Use the flow chart below to answer the questions that follow:(Solved)
Use the flow chart below to answer the questions that follow:
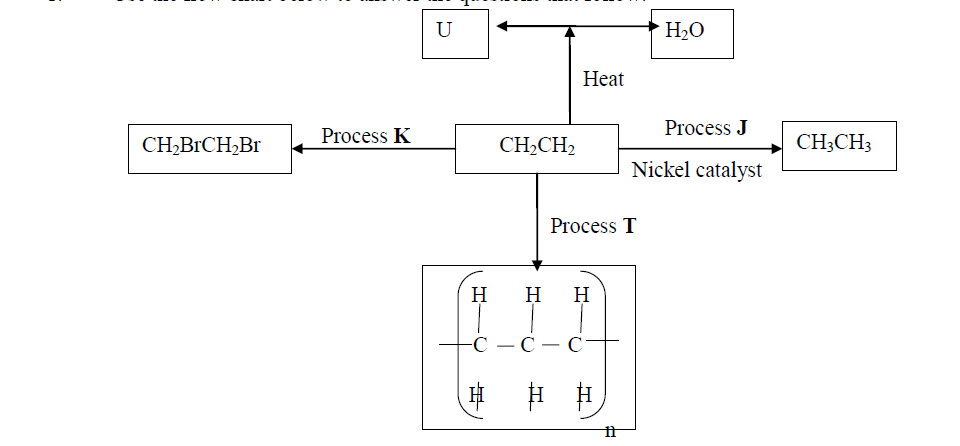
(a) What observation would be made in process K?
(b) Name another conditions necessary for process J to take place
(c) Give the name of substance V
Date posted: October 17, 2019. Answers (1)
- During welding of cracked railway lines by thermite 12.0g of oxide of iron is reduced by aluminium to 8.40g of iron. Determine the empirical formula...(Solved)
During welding of cracked railway lines by thermite 12.0g of oxide of iron is reduced by aluminium to 8.40g of iron. Determine the empirical formula of the oxide(Fe= 56.0, O= 16.0)
Date posted: October 17, 2019. Answers (1)
- 1.6g of magnesium metal is reacted with excess hydrochloric acid. Calculate the volume
of hydrogen gas produced (Molar gas volume at stp = 22.4dm3 Mg=24)(Solved)
1.6g of magnesium metal is reacted with excess hydrochloric acid. Calculate the volume
of hydrogen gas produced (Molar gas volume at stp = 22.4dm3 Mg=24)
Date posted: October 17, 2019. Answers (1)