NH3 is basic and reacts with some moles of the acid hence reduction in concentration.
maurice.mutuku answered the question on October 22, 2019 at 08:37
-
Study the flow charts below and use them to answer the questions that follow:
(Solved)
Study the flow charts below and use them to answer the questions that follow:
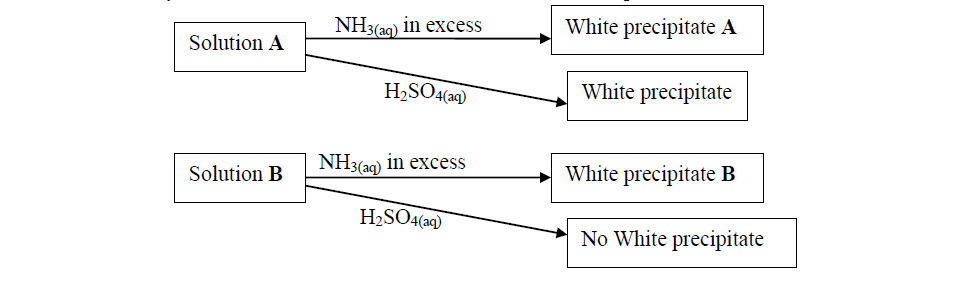
(a) Identify possible cations present in:
(i) Solution A
(ii) Solution B
(b) State and explain the observations made when a sample of dry white precipitate B is heated in a test-tube
Date posted:
October 22, 2019
.
Answers (1)
-
The diagram below is used in preparation of a gas in the laboratory. Answer the questions that follow;
(Solved)
The diagram below is used in preparation of a gas in the laboratory. Answer the questions that follow;

(a) Name gas X
(b) State one physical property which makes it possible for the gas to be collected as shown
(c) State one commercial use of gas X
Date posted:
October 22, 2019
.
Answers (1)
-
(a) Explain the importance of the high percentage of nitrogen in air(b) Why is nitrogen used for storage of semen in artificial insemination?
(Solved)
(a) Explain the importance of the high percentage of nitrogen in air
(b) Why is nitrogen used for storage of semen in artificial insemination?
Date posted:
October 22, 2019
.
Answers (1)
-
Ammonia gas is prepared in the laboratory by the action of an alkali on an ammonium salt.
A student wanted to prepare a sample of ammonia...
(Solved)
Ammonia gas is prepared in the laboratory by the action of an alkali on an ammonium salt.
A student wanted to prepare a sample of ammonia gas in the laboratory.
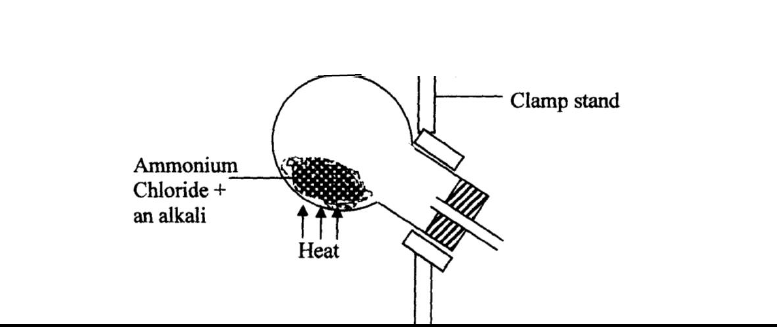
(a) Give one alkali that can be used in the above experiment
(b) Write an equation for the reaction that takes place in the above experiment
Date posted:
October 22, 2019
.
Answers (1)
-
The diagram below shows the catalytic oxidation of ammonia gas. Use it to answer the questions that follow:-
(Solved)
The diagram below shows the catalytic oxidation of ammonia gas. Use it to answer the questions that follow:-
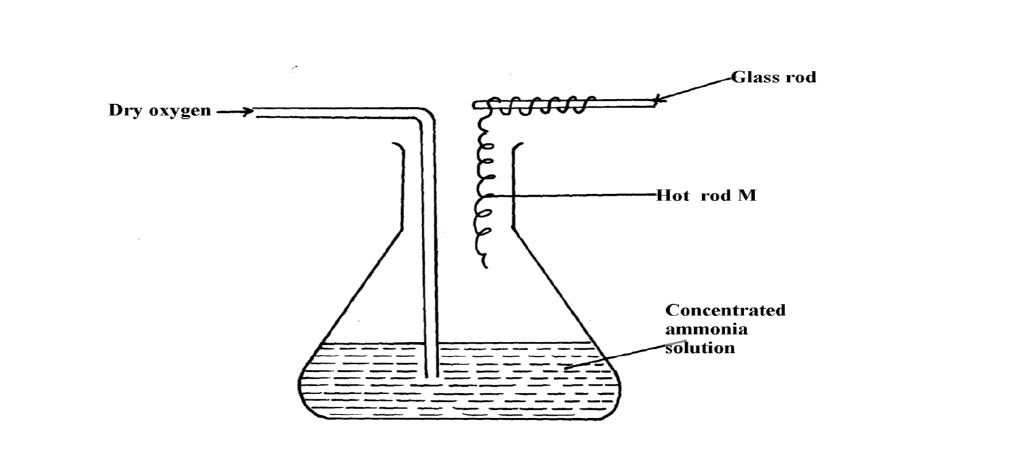
(a) What metal could rod M be made of?
(b) State and explain two observations made inside the conical flask
Date posted:
October 22, 2019
.
Answers (1)
-
A hydrocarbon compound Z decolourizes bromine liquid in the presence of light but does not decolourize acidified potassium manganate (VII). Name and draw the structural...
(Solved)
A hydrocarbon compound Z decolourizes bromine liquid in the presence of light but does not decolourize acidified potassium manganate (VII). Name and draw the structural formula of the eighth member of this homologous series
Date posted:
October 22, 2019
.
Answers (1)
-
(i) 2.63g of a solution of sodium chloride at 20.0oC was reacted with silver nitrate. After filtration,washing and drying, 2.36g of silver chloride was obtained....
(Solved)
(i) 2.63g of a solution of sodium chloride at 20.0oC was reacted with silver nitrate. After filtration,washing and drying, 2.36g of silver chloride was obtained. Determine the solubility of sodium
chloride at 20.0oC . (Na=23, Cl= 35.5, Ag = 108)
(ii) Determine the number of moles of carbon (IV) Oxide gas produced when sodium carbonate reacted with dilute sulphuric (VI) acid (Molar gas volume =24dm3)
Date posted:
October 22, 2019
.
Answers (1)
-
Substance “M” with a general formula C2Hy burnt in chlorine gas with a red flame producing a cloud of black specks and colourless gas G.
(a)...
(Solved)
Substance “M” with a general formula C2Hy burnt in chlorine gas with a red flame producing a cloud of black specks and colourless gas G.
(a) State the collective name for compounds which ‘M’ belongs
(b) With reason, state the identity of the black specks and colour gas “G”.
Date posted:
October 22, 2019
.
Answers (1)
-
Study the data given in the following table and answer the questions that follow. The letters are not the actual symbols of elements.
(Solved)
Study the data given in the following table and answer the questions that follow. The letters are not the actual symbols of elements.
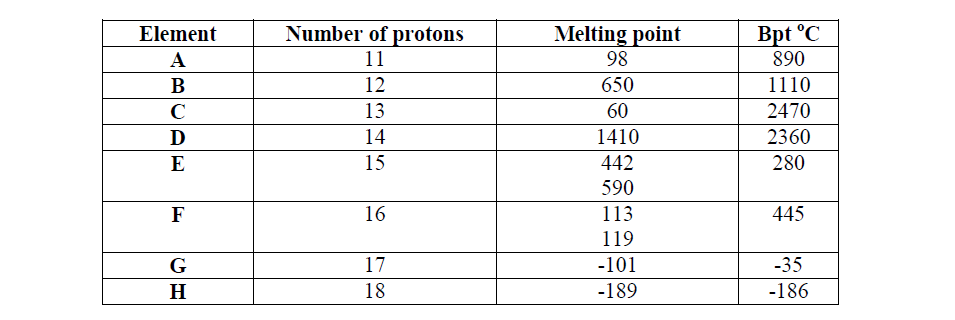
(i) State and explain the trend in melting point in A B C
(ii) Explain why the melting point and boiling points of element D is the highest
(iii) Explain why the element represented by letter E has two melting point values
(iv) Write down the chemical formula between element C and sulphate ions
(v) Name the chemical family in which H belong and state one use of the element
(vi) What is the nature of the oxide of the elements represented by letters C and F?
Date posted:
October 22, 2019
.
Answers (1)
-
Give two ways how the disposal of polymers such as polychloroethene by burning pollutes the environment
(Solved)
Give two ways how the disposal of polymers such as polychloroethene by burning pollutes the environment
Date posted:
October 22, 2019
.
Answers (1)
-
State the observations made when buton-l-ol reacts with:-
(i) Acidified potassium dichromate (VI) solution
(ii) Potassium metal
(Solved)
State the observations made when buton-l-ol reacts with:-
(i) Acidified potassium dichromate (VI) solution
(ii) Potassium metal
Date posted:
October 22, 2019
.
Answers (1)
-
The set-up below was used to prepare ethyne gas
(Solved)
The set-up below was used to prepare ethyne gas
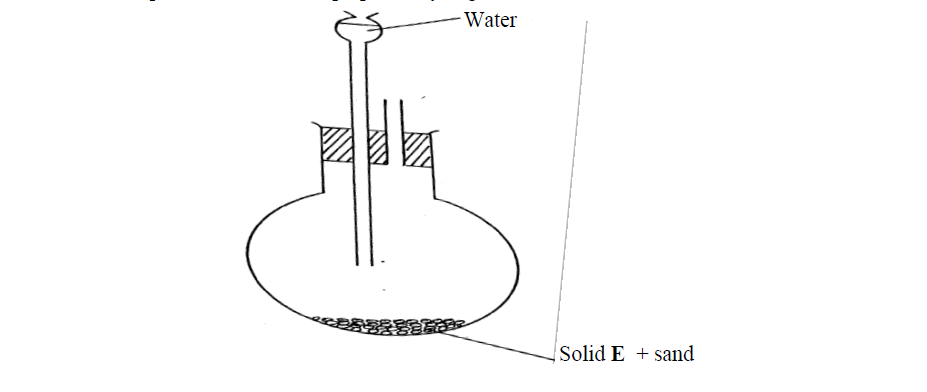
(i) Identify solid E
(ii) Complete the diagram to show how the gas can be collected
(iii) Write an equation to show how the gas is formed
(iv) Complete the equation below: )
C2H2 + 2I2---------------->
(v) What is the role of sand in the experiment?
Date posted:
October 18, 2019
.
Answers (1)
-
The diagram below represents a large-scale fractional distillation plant used to separate the components A, B, C and D in a mixture
(Solved)
The diagram below represents a large-scale fractional distillation plant used to separate the components A, B, C and D in a mixture
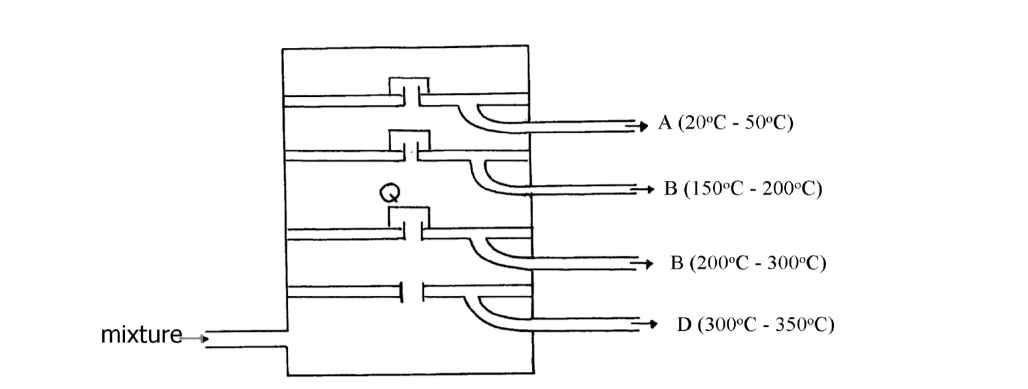
The components have the following average relative molecular masses not necessarily in that
order; 282, 184, 44 and 128.
(i) What is the physical state of B at the position marked Q?
(ii) Which component has an average relative molecular mass of 128? Explain
(iii) State with a reason whether C is pure or impure
(iv) Explain how the mixture is separated into its components
(v) Name two naturally occurring mixtures that are separated using this process
Date posted:
October 18, 2019
.
Answers (1)
-
Give the open structures of:-
(i) 3-chlorohex-l-yne
(ii) CH3OH
(Solved)
Give the open structures of:-
(i) 3-chlorohex-l-yne
(ii) CH3OH
Date posted:
October 18, 2019
.
Answers (1)
-
10cm3 of methane (CH4) gas is exploded with 150cm3 of air containing 20% oxygen and 80% nitrogen. The products were allowed to cool to room...
(Solved)
10cm3 of methane (CH4) gas is exploded with 150cm3 of air containing 20% oxygen and 80% nitrogen. The products were allowed to cool to room temperature. What will be the total volume of the gases at the end of the reaction?
Date posted:
October 17, 2019
.
Answers (1)
-
Polymerisation of ethene takes place as shown in the equation below
(Solved)
Polymerisation of ethene takes place as shown in the equation below:
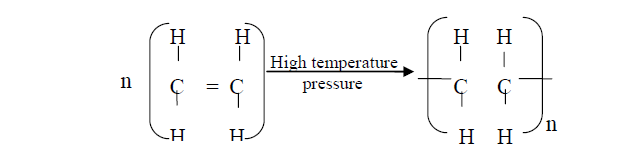
Name the type of polymerisation undergone by ethene in the reaction above
Date posted:
October 17, 2019
.
Answers (1)
-
In a reaction an alcohol K was converted to hex-1-ene
Name reagent and condition necessary for the reaction above to occur
(Solved)
In a reaction an alcohol K was converted to hex-1-ene
Name reagent and condition necessary for the reaction above to occur
Date posted:
October 17, 2019
.
Answers (1)
-
In petrol chemical industries, long chain alkanes are broken down in to simpler substances in a process called cracking
a) Why is cracking necessary?
b) State the...
(Solved)
In petrol chemical industries, long chain alkanes are broken down in to simpler substances in a process called cracking
a) Why is cracking necessary?
b) State the two conditions required in cracking
c) Draw the structure of 1-chloro-2, 2-dimethylpropane
Date posted:
October 17, 2019
.
Answers (1)
-
But-z-ene undergoes hydrogenation according to the equation given below
(Solved)
But-z-ene undergoes hydrogenation according to the equation given below
CH3CH = CHCH3(g) + H2(g)----->CH3CH2CH2CH3(g)
Name the product formed when but-z-ene reacts with hydrogen gas
Date posted:
October 17, 2019
.
Answers (1)
-
Use the flow chart below to answer the questions that follow:
(Solved)
Use the flow chart below to answer the questions that follow:
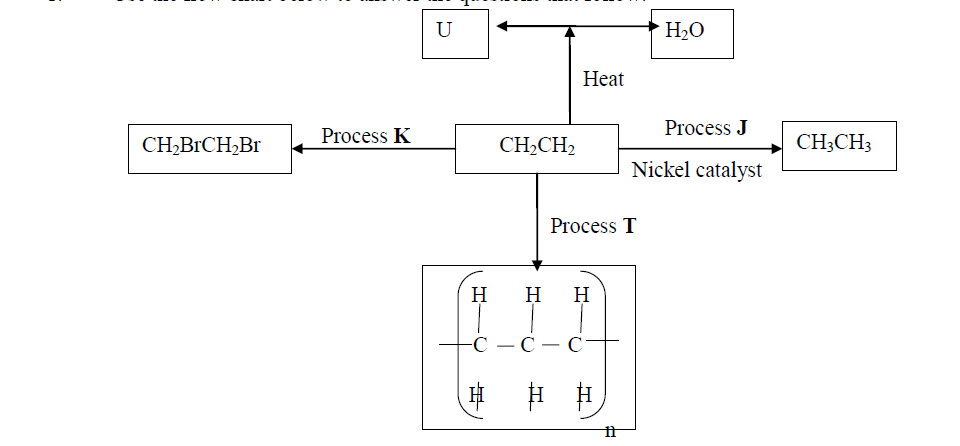
(a) What observation would be made in process K?
(b) Name another conditions necessary for process J to take place
(c) Give the name of substance V
Date posted:
October 17, 2019
.
Answers (1)