2N2O(g) + C(s)------->Co2(g) + 2N2(g)
maurice.mutuku answered the question on October 24, 2019 at 05:20
- When sulphur powder is heated to over 400oC the following changes are observed:-
At 113oC it melts into light brown liquid. The liquid then darkens to...(Solved)
When sulphur powder is heated to over 400oC the following changes are observed:-
At 113oC it melts into light brown liquid. The liquid then darkens to become reddish-brown
and very viscous at 160oC. Above 160oC the liquid becomes almost black. At the boiling point the liquid becomes mobile. Explain these observations
Date posted: October 24, 2019. Answers (1)
- The diagram below was used to investigate the reaction between nitrogen(I)oxide and copper turnings. Study it and answer the questions that follow:(Solved)
The diagram below was used to investigate the reaction between nitrogen(I)oxide and copper turnings. Study it and answer the questions that follow:

a) What has been omitted in the set-up?
b) Write a chemical equation for the reaction that took place in the combustion tube
c) State one use of gas P
Date posted: October 24, 2019. Answers (1)
- The diagram below represents an in complete set-up for preparation of a dry sample of gas R(Solved)
The diagram below represents an in complete set-up for preparation of a dry sample of gas R

a) Complete the set-up to show how a dry sample of gas R is collected
b) Write a chemical equation for the reaction that produces gas R
Date posted: October 22, 2019. Answers (1)
- The diagram below is a set-up used in preparation of ammonia solution. Study it and answer the questions that follow(Solved)
The diagram below is a set-up used in preparation of ammonia solution. Study it and answer the questions that follow

(i) What is the purpose of the filter funnel in the set-up above?
(ii) What would happen if a delivery tube was used in place of the filter funnel?
(iii) What observation would be made on litmus paper placed into the solution in the beaker at the end of the experiment?
Date posted: October 22, 2019. Answers (1)
- The diagram below is a set-up for preparation and collection of a gas. Study it answer the questions that follow:(Solved)
The diagram below is a set-up for preparation and collection of a gas. Study it answer the questions that follow:

(i) Identify gas X
(ii) Write an equation for the formation of gas X
(iii) What precaution should be observed when preparing gas X by the above method?
(iv) Describe the suitable drying agent for gas X
(v) How can one confirm that the gas collected is gas X?
(vi) State two physical properties of gas X
Date posted: October 22, 2019. Answers (1)
- The chart below shows a summary for the preparation of nitrogen gas from air(Solved)
The chart below shows a summary for the preparation of nitrogen gas from air

(a) What is the purpose of the sodium hydroxide?
(b) Write an equation for the reaction taking place in chamber II
(c) The nitrogen gas obtained is not pure. Explain
Date posted: October 22, 2019. Answers (1)
- A mixture of N2, H2 and NH3 was bubbled through 0.2M hydrochloric acid solution.
The final concentration of the acid was found to be 0.1M. Give...(Solved)
A mixture of N2, H2 and NH3 was bubbled through 0.2M hydrochloric acid solution.
The final concentration of the acid was found to be 0.1M. Give explanation
Date posted: October 22, 2019. Answers (1)
- Study the flow charts below and use them to answer the questions that follow:(Solved)
Study the flow charts below and use them to answer the questions that follow:
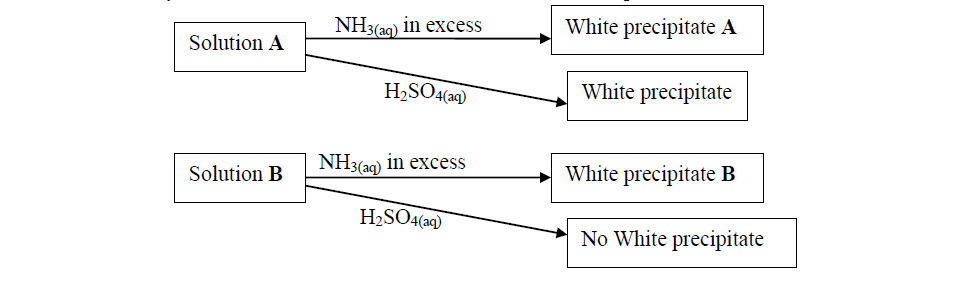
(a) Identify possible cations present in:
(i) Solution A
(ii) Solution B
(b) State and explain the observations made when a sample of dry white precipitate B is heated in a test-tube
Date posted: October 22, 2019. Answers (1)
- The diagram below is used in preparation of a gas in the laboratory. Answer the questions that follow;(Solved)
The diagram below is used in preparation of a gas in the laboratory. Answer the questions that follow;

(a) Name gas X
(b) State one physical property which makes it possible for the gas to be collected as shown
(c) State one commercial use of gas X
Date posted: October 22, 2019. Answers (1)
- (a) Explain the importance of the high percentage of nitrogen in air(b) Why is nitrogen used for storage of semen in artificial insemination?(Solved)
(a) Explain the importance of the high percentage of nitrogen in air
(b) Why is nitrogen used for storage of semen in artificial insemination?
Date posted: October 22, 2019. Answers (1)
- Ammonia gas is prepared in the laboratory by the action of an alkali on an ammonium salt.
A student wanted to prepare a sample of ammonia...(Solved)
Ammonia gas is prepared in the laboratory by the action of an alkali on an ammonium salt.
A student wanted to prepare a sample of ammonia gas in the laboratory.
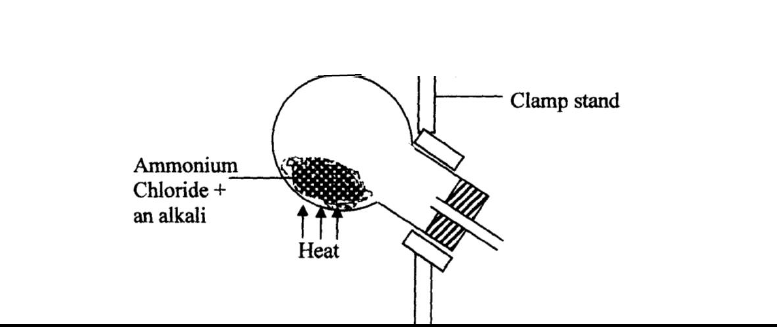
(a) Give one alkali that can be used in the above experiment
(b) Write an equation for the reaction that takes place in the above experiment
Date posted: October 22, 2019. Answers (1)
- The diagram below shows the catalytic oxidation of ammonia gas. Use it to answer the questions that follow:-(Solved)
The diagram below shows the catalytic oxidation of ammonia gas. Use it to answer the questions that follow:-
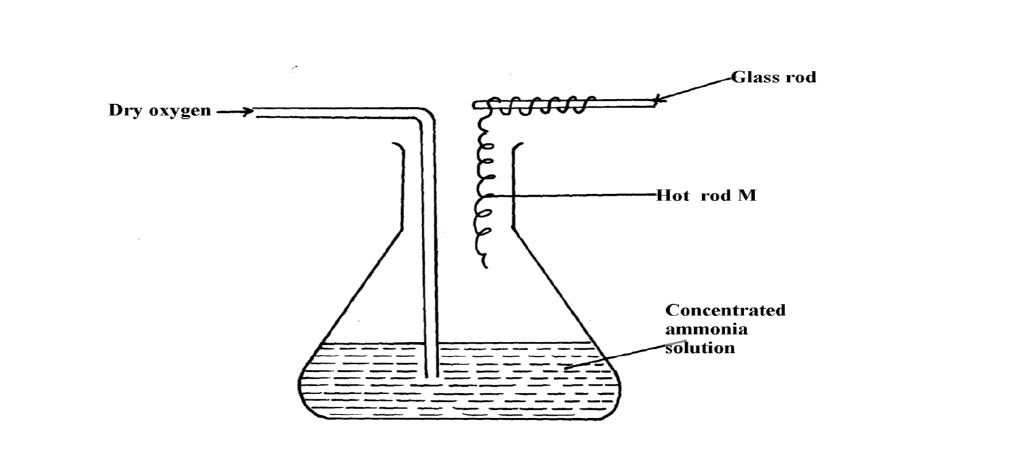
(a) What metal could rod M be made of?
(b) State and explain two observations made inside the conical flask
Date posted: October 22, 2019. Answers (1)
- A hydrocarbon compound Z decolourizes bromine liquid in the presence of light but does not decolourize acidified potassium manganate (VII). Name and draw the structural...(Solved)
A hydrocarbon compound Z decolourizes bromine liquid in the presence of light but does not decolourize acidified potassium manganate (VII). Name and draw the structural formula of the eighth member of this homologous series
Date posted: October 22, 2019. Answers (1)
- Write down all the isomers of but-z-ene and give their IUPAC names(Solved)
Write down all the isomers of but-z-ene and give their IUPAC names
Date posted: October 22, 2019. Answers (1)
- (i) 2.63g of a solution of sodium chloride at 20.0oC was reacted with silver nitrate. After filtration,washing and drying, 2.36g of silver chloride was obtained....(Solved)
(i) 2.63g of a solution of sodium chloride at 20.0oC was reacted with silver nitrate. After filtration,washing and drying, 2.36g of silver chloride was obtained. Determine the solubility of sodium
chloride at 20.0oC . (Na=23, Cl= 35.5, Ag = 108)
(ii) Determine the number of moles of carbon (IV) Oxide gas produced when sodium carbonate reacted with dilute sulphuric (VI) acid (Molar gas volume =24dm3)
Date posted: October 22, 2019. Answers (1)
- Substance “M” with a general formula C2Hy burnt in chlorine gas with a red flame producing a cloud of black specks and colourless gas G.
(a)...(Solved)
Substance “M” with a general formula C2Hy burnt in chlorine gas with a red flame producing a cloud of black specks and colourless gas G.
(a) State the collective name for compounds which ‘M’ belongs
(b) With reason, state the identity of the black specks and colour gas “G”.
Date posted: October 22, 2019. Answers (1)
- Study the data given in the following table and answer the questions that follow. The letters are not the actual symbols of elements.(Solved)
Study the data given in the following table and answer the questions that follow. The letters are not the actual symbols of elements.
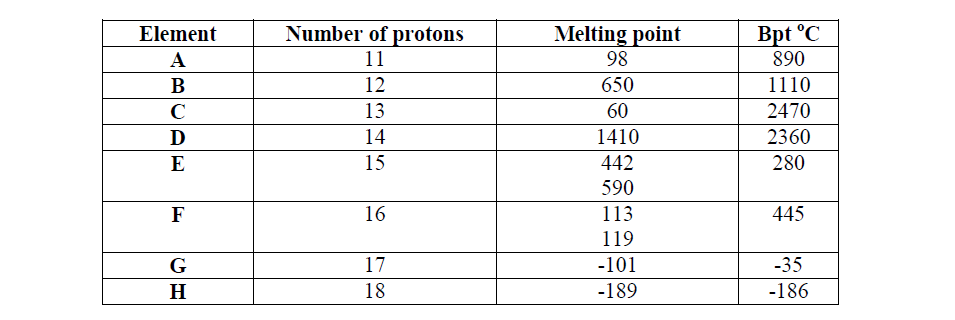
(i) State and explain the trend in melting point in A B C
(ii) Explain why the melting point and boiling points of element D is the highest
(iii) Explain why the element represented by letter E has two melting point values
(iv) Write down the chemical formula between element C and sulphate ions
(v) Name the chemical family in which H belong and state one use of the element
(vi) What is the nature of the oxide of the elements represented by letters C and F?
Date posted: October 22, 2019. Answers (1)
- Give the systematic names of the following compounds;(Solved)
Give the systematic names of the following compounds;

Date posted: October 22, 2019. Answers (1)
- Study the scheme below and answer the questions that follow:(Solved)
Study the scheme below and answer the questions that follow:

Name the reagents in
Step I
Step II
Step IV
(ii) Write an equation for the complete combustion of 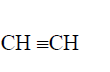
(iii) Give two uses of CH4
Date posted: October 22, 2019. Answers (1)
- Ethene is used in making polyethene bag in a process called polymerization
(i) Name the type of polymer that is formed when ethane polymerise
(ii) Describe a...(Solved)
Ethene is used in making polyethene bag in a process called polymerization
(i) Name the type of polymer that is formed when ethane polymerise
(ii) Describe a simple chemical test that can be used to identify ethane gas in the laboratory
Date posted: October 22, 2019. Answers (1)