Put the mixture in a beaker, add water and stir. Copper (II) sulphate dissolves while iron (III) oxide does not. Filter the mixture to obtain copper (II) sulphate solution as the filtrate and iron (III) oxide as the residue. Wash the residue with distilled water and dry it between two filter papers.
johnmulu answered the question on February 2, 2017 at 05:18
- Describe how samples of Lead (II) Sulphate, ammonium chloride and sodium chloride can be obtained from a mixture of the three (Solved)
Describe how samples of Lead (II) Sulphate, ammonium chloride and sodium chloride can be obtained from a mixture of the three.
Date posted: February 2, 2017. Answers (1)
- When solid A was heated strongly, it gave off water residue. When water was added to the solid residue, the original solid A, was formed (Solved)
When solid A was heated strongly, it gave off water residue. When water was added to the solid residue, the original solid A, was formed
a) What name is given to the process described
b) Give one example of solid A
Date posted: February 2, 2017. Answers (1)
- Describe an experimental procedure that can be used to extract oil from nut seeds.(Solved)
Describe an experimental procedure that can be used to extract oil from nut seeds.
Date posted: February 1, 2017. Answers (1)
- A sample of water in a beaker was found to boil at 101o C at 1 atmospheric pressure.(Solved)
A sample of water in a beaker was found to boil at 101o C at 1 atmospheric pressure. Assuming that the thermometer was not faulty, explain this observation.
Date posted: February 1, 2017. Answers (1)
- A mixture contains ammonium chloride, copper (II) oxide and sodium chloride. Describe how each of the substances can be obtained from the mixture.(Solved)
A mixture contains ammonium chloride, copper (II) oxide and sodium chloride. Describe how each of the substances can be obtained from the mixture.
Date posted: January 31, 2017. Answers (1)
- Describe a laboratory experiment that can be used to show that the action of heat on hydrated cobalt (II) chloride is a reversible action.(Solved)
Hydrated cobalt (II) chloride exists as pink crystals and anhydrous cobalt (II) chloride is a blue powder. Describe a laboratory experiment that can be used to show that the action of heat on hydrated cobalt (II) chloride is a reversible action.
Date posted: January 31, 2017. Answers (1)
- State and explain the change in mass that occurs when the following substances are separately heated in open crucibles(Solved)
State and explain the change in mass that occurs when the following substances are separately heated in open crucibles.
(a) Copper metal
(b) Copper (II) nitrate
Date posted: January 31, 2017. Answers (1)
- Name the regions labeled C and D in the Bunsen burner below(Solved)
The diagram below shows a Bunsen burner when in use.
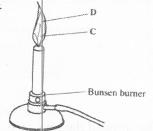
Date posted: January 28, 2017. Answers (1)
- What are the differences between luminous and no-luminous flames?(Solved)
What are the differences between luminous and no-luminous flames?
Date posted: January 28, 2017. Answers (1)
- State and explain two reasons why hydrogen is very attractive fuel compared to fossils(Solved)
Natural gas, coal and oil are known as fossil fuels. They are major sources of energy. Hydrogen gas is also a source of energy. State and explain two reasons why hydrogen is very attractive fuel compared to fossils.
Date posted: January 16, 2017. Answers (1)
- What is a fuel?(Solved)
Define what a fuel is.
Date posted: January 16, 2017. Answers (1)
- What is molar heat of vaporization(Solved)
What is molar heat of vaporization? Explain.
Date posted: January 16, 2017. Answers (1)
- Explain why graphite can be used as a lubricant while diamond cannot(Solved)
Explain why graphite can be used as a lubricant while diamond cannot.
Date posted: January 16, 2017. Answers (1)
- State two non crystalline (Amorphous) form of carbon(Solved)
Diamond and graphite are crystalline forms of cation. State two non crystalline (Amorphous) form of carbon.
Date posted: January 16, 2017. Answers (1)
- State two observations made when excess hydrogen suphide gas is bubbled into copper (II) sulphate solution(Solved)
State observations made when excess hydrogen suphide gas is bubbled into copper (II) sulphate solution.
Date posted: January 16, 2017. Answers (1)
- A student attempted to prepare hydrogen sulphide gas and collected the gas over cold water(Solved)
A student attempted to prepare hydrogen sulphide gas and collected the gas over cold water. The student collected only a few bubbles of gas. Explain.
Date posted: January 16, 2017. Answers (1)
- Describe how a pure sample of zinc sulphate can be prepared in the laboratory starting with zinc oxide(Solved)
Starting with zinc oxide, describe how a pure sample of zinc sulphate can be prepared in the laboratory.
Date posted: January 16, 2017. Answers (1)
- A form three student added sodium carbonate solution to a solution of aluminium chloride in water(Solved)
A form three student added sodium carbonate solution to a solution of aluminium chloride in water. A white precipitate was formed. The precipitate soon dissolved liberation a colourless gas.
i) What is the white precipitate?
ii) Explain why the precipitate dissolves?
Date posted: January 16, 2017. Answers (1)
- Magnesium and silicon are both in period III of the periodic table(Solved)
Magnesium and silicon are both in period III of the periodic table. The atomic radius of the magnesium is found to be higher than silicon. Explain this observation.
Date posted: January 16, 2017. Answers (1)
- Nitrogen (II) oxide is produced in the internal combustion engines(Solved)
Nitrogen (II) oxide is produced in the internal combustion engines. State and explain how it affects the environment.
Date posted: January 16, 2017. Answers (1)