a) To remove any oxide film on it
b) A white solid formed which is magnesium oxide
c) The increase in mass was due to the oxygen which combines with magnesium
johnmulu answered the question on February 2, 2017 at 07:04
- When wood is burnt, a grey powder called ash remains. The ash is stirred with water and filtered a colourless solution is obtained(Solved)
When wood is burnt, a grey powder called ash remains. The ash is stirred with water and filtered a colourless solution is obtained
a) What is the main component of the colourless solution
b) Explain your answer in (a) above
c) State the observation that would be made if methyl orange indicator was passed through the solution of ash.
Date posted: February 2, 2017. Answers (1)
- Name another gas which used together with oxygen in welding(Solved)
Name another gas which used together with oxygen in welding
Date posted: February 2, 2017. Answers (1)
- When magnesium metal is burnt in air, it reacts with both oxygen and nitrogen gases giving a white ash(Solved)
When magnesium metal is burnt in air, it reacts with both oxygen and nitrogen gases giving a white ash. Write two equations for the reactions that take place
Date posted: February 2, 2017. Answers (1)
- Explain how you would separate a mixture of nitrogen and oxygen gases given that their boiling points are(Solved)
Explain how you would separate a mixture of nitrogen and oxygen gases given that their boiling points are - 196o C and - 183o C
Date posted: February 2, 2017. Answers (1)
- (a) Name a suitable solvent for extracting an indicator from flowers (b) Give a reason why the solvent named in (a) above is used (Solved)
a) Name a suitable solvent for extracting an indicator from flowers
b) Give a reason why the solvent named in (a) above is used
Date posted: February 2, 2017. Answers (1)
- Describe how pH of anti-acid (Actal) powder can be determined in the laboratory(Solved)
Describe how pH of anti-acid (Actal) powder can be determined in the laboratory
Date posted: February 2, 2017. Answers (1)
- Starting with red roses, describe how (i) a solution containing the red pigment may be prepared(Solved)
Starting with red roses, describe how;
i) a solution containing the red pigment may be prepared
ii) The solution can be shown to be an indicator
Date posted: February 2, 2017. Answers (1)
- When a student was stung by a nettle plant, a teacher applied an aqueous solution of ammonia to the affected area(Solved)
When a student was stung by a nettle plant, a teacher applied an aqueous solution of ammonia to the affected area of the skin and the student was relieved of pain. Explain
Date posted: February 2, 2017. Answers (1)
- The pH of a sample of soil was found to be 5.0. An agricultural officer recommended the addition of calcium oxide in the soil(Solved)
The pH of a sample of soil was found to be 5.0. An agricultural officer recommended the addition of calcium oxide in the soil. State two functions of the calcium oxide in the soil
Date posted: February 2, 2017. Answers (1)
- 10g of sodium hydrogen carbonate were dissolved in 20 cm3 of water in a boiling tube. Lemon juice was then added dropwise with shaking unit there was no further observable change (Solved)
10g of sodium hydrogen carbonate were dissolved in 20 cm3 of water in a boiling tube. Lemon juice was then added dropwise with shaking unit there was no further observable change
a) Explain the observation which was made in boiling tube when the reaction was in progress
b) What observation would have been made if the lemon juice had been added to copper turning in a boiling tube? Give a reason
Date posted: February 2, 2017. Answers (1)
- A mixture contains ammonium chloride, copper (II) oxide and sodium chloride. Describe how each of the substances can be obtained from the mixture(Solved)
A mixture contains ammonium chloride, copper (II) oxide and sodium chloride. Describe how each of the substances can be obtained from the mixture
Date posted: February 2, 2017. Answers (1)
- Iron (III) oxide was found to be contaminated with copper (II) sulphate. Describe how a pure sample of iron (III) oxide can be obtained(Solved)
Iron (III) oxide was found to be contaminated with copper (II) sulphate. Describe how a pure sample of iron (III) oxide can be obtained.
Date posted: February 2, 2017. Answers (1)
- Describe how samples of Lead (II) Sulphate, ammonium chloride and sodium chloride can be obtained from a mixture of the three (Solved)
Describe how samples of Lead (II) Sulphate, ammonium chloride and sodium chloride can be obtained from a mixture of the three.
Date posted: February 2, 2017. Answers (1)
- When solid A was heated strongly, it gave off water residue. When water was added to the solid residue, the original solid A, was formed (Solved)
When solid A was heated strongly, it gave off water residue. When water was added to the solid residue, the original solid A, was formed
a) What name is given to the process described
b) Give one example of solid A
Date posted: February 2, 2017. Answers (1)
- Describe an experimental procedure that can be used to extract oil from nut seeds.(Solved)
Describe an experimental procedure that can be used to extract oil from nut seeds.
Date posted: February 1, 2017. Answers (1)
- A sample of water in a beaker was found to boil at 101o C at 1 atmospheric pressure.(Solved)
A sample of water in a beaker was found to boil at 101o C at 1 atmospheric pressure. Assuming that the thermometer was not faulty, explain this observation.
Date posted: February 1, 2017. Answers (1)
- A mixture contains ammonium chloride, copper (II) oxide and sodium chloride. Describe how each of the substances can be obtained from the mixture.(Solved)
A mixture contains ammonium chloride, copper (II) oxide and sodium chloride. Describe how each of the substances can be obtained from the mixture.
Date posted: January 31, 2017. Answers (1)
- Describe a laboratory experiment that can be used to show that the action of heat on hydrated cobalt (II) chloride is a reversible action.(Solved)
Hydrated cobalt (II) chloride exists as pink crystals and anhydrous cobalt (II) chloride is a blue powder. Describe a laboratory experiment that can be used to show that the action of heat on hydrated cobalt (II) chloride is a reversible action.
Date posted: January 31, 2017. Answers (1)
- State and explain the change in mass that occurs when the following substances are separately heated in open crucibles(Solved)
State and explain the change in mass that occurs when the following substances are separately heated in open crucibles.
(a) Copper metal
(b) Copper (II) nitrate
Date posted: January 31, 2017. Answers (1)
- Name the regions labeled C and D in the Bunsen burner below(Solved)
The diagram below shows a Bunsen burner when in use.
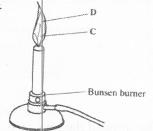
Date posted: January 28, 2017. Answers (1)